Pubertal Administration of Angiotensin Receptor Blocker Modifies Renal Vascular Structural Alterations in Dahl Salt-sensitive Rats (Effect of ARB on Kidney of Dahl Salt-sensitive Rat)
Hexing L,Tomoda F, Koike T, Hidenori Y, Ohara M and Inoue H
Hexing L1, Tomoda F2, Koike T1, Yamazaki H1, Ohara M1 and Inoue H3
1The Second Department of Internal Medicine, University of Toyama, Medical Stuff, Toyama, Japan
2Fukui College of Health Sciences, Fukui, Japan
3Saiseikai Toyama Hospital, Director of a Hospital, Toyama, Japan
- Corresponding Author:
- Fumihiro Tomoda
Professor, Fukui College of Health Sciences
Fukui-City, Fukui 910-3190, Japan
Tel: 81 776 59 2200
E-mail: fcm-tomoda@khc.biglobe.ne.jp
Received April 04, 2015; Accepted April 12, 2015; Submitted April 25, 2016
Citation: Hexing L, Tomoda F, Koike T, et al. Pubertal Administration of Angiotensin Receptor Blocker Modifies Renal Vascular Structural Alterations in Dahl Salt-sensitive Rats (Effect of ARB on Kidney of Dahl Saltsensitive Rat). Insights Blood Press 2016, 2:1.
Abstract
Salt-induced renal vascular structural changes and their alterations by pubertal treatment with an angiotensin II receptor blocker, olmesartan, were examined in Dahl salt-sensitive (DS) rats using Dahl salt-resistant (DR) rats as controls (n=24 of each strain). DS and DR rats were treated with olmesartan (10 mg/kg/day) or vehicle for two weeks starting from 3 to 4 weeks of age and thereafter fed 4% salt diet (n=8 of each treatment in each strain). The remainders of both strains were fed 0.3% salt diet throughout the study period. At 10-12 weeks of age, flowpressure (F-P) and pressure-glomerular filtration rate (P-GFR) relationships were determined for maximally vasodilated, perfused kidneys. In the rats receiving 0.3% salt diet, the gradient of P-GFR (glomerular filtration capacity against pressure) was less in DS strain than in DR strain, although blood pressure, the gradient of F-P (minimal renal vascular resistance reflecting the overall luminal dimensions of preand post-glomerular vasculature) and the X intercept of P-GFR (preglomerular: postglomerular vascular resistance ratio) did not differ between both strains. In DS strain, blood pressure were higher, the gradient of F-P was greater and the gradient of P-GFR was less in the rats receiving 4% salt diet plus vehicle compared with the rats receiving 0.3% salt diet, although the X intercept of P-GFR did not differ between the two groups. Contrary, the above parameters were not different between the two groups in DR strain. In DS strain, blood pressure was lower and the gradient of P-GFR was greater in the rats receiving 4% salt diet plus olmesartan compared with the rats receiving 4% salt diet plus vehicle, although the gradient of F-P and X intercept of P-GFR did not differ between the two groups. Conversely, the above parameters were not different between the two groups in DR strain. In conclusion, the pubertal treatment with olmesartan could prevent the saltinduced worsening of reduction in glomerular filtration capacity as well as saltinduced increase in blood pressure in DS strain.
Keywords
Dahl salt-sensitive rats; Angiotensin II receptor blocker; Olmesartan; Renal vascular structure; Pubertal period
Introduction
In salt-sensitive hypertension, defined by an increased susceptibility to the blood-pressure-raising effects of salt [1], high salt intake increases not only systemic blood pressure but also intraglomerular pressure [2-5]. Because intraglomerular hypertension plays an important role in the genesis of glomerular injuries [6], the intrarenal hemodynamic alteration induced by salt loading might be responsible for the early onset and rapid progression of renal damage in salt-sensitive hypertension [8-11]. In salt-sensitive hypertension, therefore, elucidation of mechanisms underlying the renal hemodynamic responses to salt loading would be useful for devising strategies for prevention of renal injuries.
In hypertension, resistance vessels become thicker or encroach into the lumen, i.e., vascular hypertrophy or vascular remodeling, in kidneys as well as in the other vascular beds [11,12]. These vascular structural alterations could affect regional hemodynamics of organs including the kidney, as proposed by Folkow and Korner [11-13]. In fact, wall-thickening and vascular narrowing occurs in renal preglomerular resistance vessels of spontaneously hypertensive rats (SHR), one of the non-salt-sensitive hypertensive models [13]. This renal vascular structural change could lead to exaggerated vasoconstriction even if the vasoactive stimuli are constant, and thereby maintain normal glomerular pressure in the face of systemic hypertension in SHR. Therefore, renal vascular structural geometry may be essential for the regulation of intraglomerular hemodynamics in hypertension. However, little evidence is available concerning renal vascular structure associated with the genesis of intraglomerular hypertension in salt-sensitive hypertension [14].
In SHR, the administration of angiotensin converting enzyme (ACE) inhibitors or angiotensin II receptor blockers (ARB) during the pubertal period (3 to 10 weeks of age) at the prehypertensive stage can attenuate the later development of hypertension [15,16]. Additionally, in SHR, brief treatment with ACE inhibitors for two weeks is effective in causing a sustained reduction of blood pressure even if the drug administration was started at age of 16 weeks, when hypertension was established [17]. Interestingly, a decrease in structurally based renal vascular resistance also occurred in the kidney of treated SHR and the prolonged hypotensive effect of ACE inhibitors could be transferred from treated SHR to untreated SHR by cross-transplantation of kidneys [17]. Thus, in SHR, the persistent lowering of blood pressure following the transient inhibition of the renin angiotensin system (RAS) at prehypertensive or early hypertensive stage could be attributed to kidney-specific vascular structural changes. Similarly, in Dahl salt-sensitive (DS) rats, one of the salt-sensitive hypertensive models, the short-term treatment of ARB during pubertal period leads to attenuation of the later development of saltinduced hypertension and glomerular damages [18]. However, it has not yet been elucidated in DS rats whether transient inhibition of the RAS during pubertal period can modulate the later development of salt-induced renal vascular structural changes and thereby affects progression of hypertension and renal damage.
The present study was therefore designed to investigate alterations in the renal vasculature following salt loading in DS rats. Influences of brief treatment with ARB during pubertal period on salt-induced changes of renal vascular structures, possibly associated with the genesis of hypertension and hypertensive renal damages, were also determined in DS rats.
Methods
Animals and preparations
The study design and experimental protocols were in accordance with our institutional guidelines for animal research and the present study was approved by the Animal Experiment Committee of the University of Toyama. Three-week-old male DS rats and Dahl salt-resistant (DR) rats, derived from Mollegaard Breeding Center, were purchased (n=24 of each strain) and initially fed 0.3% salt (i.e., normal salt) diet. For two weeks starting from 3 to 4 weeks of age, i.e., at pubertal period, 10 mg kg-1 day-1 of olmesartan (Sankyo Co. Ltd., Tokyo, Japan) or vehicle was administered by gavage (n=8 for each treatment in both strains) and thereafter the diet was switched to 4% salt (i.e., high salt) diet until the end of the experiment in these animals. The remaining rats (n=8 for each strain) were kept on normal salt diet throughout the study period. The diet and tap water were supplied ad libitum. All rats were housed in a room maintained at constant temperature (23-25 °C) with a 12-hour light/dark cycle until the perfusion experiments. At 10-12 weeks of age, each rat was subjected to the experiments described below.
Study protocol
On the day before the perfusion experiments, 24 h urine samples were collected for measurements of urinary protein and creatinine levels. The ratio of urinary protein (mg dL-1) to creatinine (mg dL-1) concentration was determined for evaluation of urinary protein excretion [19].
The procedure for in vitro measurements in maximally vasodilated, perfused kidneys has been reported elsewhere in detail [14,20,21]. Briefly, the rats were anesthetized with pentobarbital sodium. After a catheter was inserted into the tail artery for measurement of blood pressure, a midline abdominal incision was performed. Thereafter, the intestines and adrenal glands were removed, and the abdominal aorta was isolated 1 cm proximally and distally to the left renal artery. All visible branches from the isolated aorta were ligated except for the left renal artery and mesenteric artery. The left ureter and mesenteric artery were cannulated to allow urine collection and measurement of aortic pressure close to the left renal artery (i.e., renal arterial inflow pressure), respectively. Following intravenous heparinization (3000 U kg-1), a catheter connected to the perfusion set-up was inserted retrogradely through the abdominal aorta to a position distal to the left kidney. The aorta was tied off just above the mesenteric artery to make a closed arterial circuit including the left kidney, and the left renal vein was cut. Perfusion of the left kidney was commenced with oxygenated (95% O2 and 5% CO2) artificial perfusate at room temperature (20 to 23 °C) using a peristaltic pump (Advantec Tokyo Kaisha Ltd, Tokyo, Japan) with the flow rate initially maintained at 2 ML min-1. The perfusate used was a modified Tyrode’s solution supplemented with 20 g L-1 dextran (molecular weight = 65,000, Sigma Chemical Co., St Louis, Missouri, U.S.A.) 0.9 mmol L-1 sodium nitroprusside (Sigma Chemical Co), 10 mg L-1 furosemide (Hoechst Marion Roussel Ltd., Tokyo, Japan) and 1 g L-1 inulin (Inutest, Laevosan, Lintz, Austria). The amounts of nitroprusside and furosemide used in the present study have been confirmed to keep the kidneys maximally vasodilated and inhibit tubuloglomerular feedback [14]. Rats were killed with an overdose of pentobarbital, and the renal capsule was removed to minimize tissue pressure increments during renal perfusion. For intrarenal tissue pressure measurement, a needle (25 gauge, inner diameter 0.3 mm) was inserted perpendicular to the renal surface and the tip was advanced to the renal cortex.
Urine collection and measurement of arterial inflow pressure and intrarenal tissue pressure were repeated during 7-8 stepwise increments in perfusion flow from 3 to 12 mL min-1 starting from 30 min after the initiation of renal perfusion. Urine volume was determined gravimetrically. Inulin concentrations in the perfusate and urine were measured using the anthrone method [22], and glomerular filtration rate (GFR) was calculated as the clearance of inulin.
After the final measurements, the perfusate was changed to a fixative containing 2% formaldehyde and 0.5% glutaraldehyde in 0.075 mol L-1 phosphate buffer (pH=7.2), with which the kidneys were perfused at a rate of 3 ML min-1 for 60 min. As in our previous study, perfusion of the fixative did not alter the perfusion pressure in this preparation [14,20,21], suggesting no effect on the relaxed status of the vasculature. The left kidney was then removed and placed in 7.4% formaldehyde, embedded in paraffin, sectioned and stained with periodic acid-Schiff (PAS).
Renal hemodynamics and histological appearance in the perfused kidneys
In the maximally vasodilated, perfused kidneys, neurohumoral and active autoregulatory control systems are assumed to be inoperative; therefore, the analysis of hemodynamic behavior enables us to perform the following functional assessments of structural properties in the renal resistance vessels [14,20,21]. In the flow–pressure relationship, the gradient is the renal vascular resistance at maximal dilatation (i.e., minimal renal vascular resistance), which is an accurate index of averaged overall lumen dimensions of the preglomerular and postglomerular vasculature. In the pressure-GFR relationship, the intercept of the line with the pressure axis (i.e., the threshold pressure for commencing filtration) and the gradient of the line reflect preglomerular: postglomerular vascular resistance ratio and glomerular filtration capacity against pressure, respectively.
To estimate the above-mentioned three functional parameters, the flow–pressure and pressure–GFR relationships were established by the use of the perfusion flow, arterial distending pressure and GFR at each flow rate in the present study. In the relationships, arterial distending pressure (i.e., transmural pressure) calculated as arterial inflow pressure minus renal tissue pressure, was adopted as pressure, because tissue pressure built up during the perfusion, thereby inhibiting distension of the vasculature.
Renal morphological analysis was undertaken by measuring the cross-sectional areas of the wall and lumen in the proximal interlobular arteries and the glomerular tuft areas in the superficial and juxtamedullary glomeruli using a digitizing tablet (SD-510C, Wacom Co., Tokyo, Japan) under light microscopy. These measurements were performed in 15 to 20 vessels, 50 to 60 superficial glomeruli and 30 to 50 juxtamedullary glomeruli per animal. The proximal interlobular arteries were defined as arteries within the inner cortex, branching for 500 μm from the arcuate arteries at the corticomedullary junction [14,20,21]. The wall-to-lumen ratio was calculated by dividing the crosssectional area of the vessel wall by that of the lumen. Glomerular sclerosis was assessed using glomerular sclerosis score graded by 0, 1, 2, 3 or 4 corresponding to normal, less than 25%, 25- 50%, 50-75%, or over 75% cross-sectional sclerosis, respectively [8]. The sclerosis index for each rat was calculated as follows; (N1×1+N2×2+N3×3+N4×4) n-1×100, where N1, N2, N3, and N4 represented the numbers of glomeruli exhibiting grade 1, 2, 3, and 4, respectively, and n, the number of glomeruli assessed. The scores obtained by two investigators were averaged to represent each animal.
Statistical Analysis
All data are expressed as mean ± SEM. Renal hemodynamic variables are expressed as being divided by wet weight of the right kidney. Linearity of flow-pressure and pressure-GFR relationships was examined in all individual experiments using the Pearson correlation coefficient in simple regression analysis. The gradient and X intercept of these relationships were derived from regression lines of each animal. One-way analysis of variance (ANOVA) was used for comparisons among the groups in each strain. Comparisons of the changes induced by salt loading or treatment between strains were tested by two-way ANOVA, followed by Bonferroni correction test. A P value less than 0.05 was considered statistically significant.
Results
Systolic blood pressure, kidney weight and urinary protein excretion
In the rats receiving normal salt diet, body weight and right kidney wet weight were greater in DS strain than in DR strain (Table 1). However, there were no significant differences in mean arterial pressure and pulse rate between both strains. Urinary protein to creatinine ratio in DS strain was 4 times as high as that in DR strain.
| Variables | Dahl salt-sensitive rats | Dahl salt-resistant rats | Strain×salt loading interaction by two way ANOVA | ||
|---|---|---|---|---|---|
| 0.3% salt diet (n=8) | 4.0% salt diet and vehicle (n=8) | 0.3% salt diet (n=8) | 4.0% salt diet and vehicle (n=8) | P value | |
| BW (g) | 393 ± 6† | 342 ± 9* | 332 ± 5 | 302 ± 7* | NS |
| Mean arterial pressure (mmHg) | 126 ± 7 | 147 ± 6* | 122 ± 3 | 112 ± 5 | P<0.01 |
| Pulse rate (beats/min) | 436 ± 23 | 358 ± 16* | 404 ± 12 | 312 ± 8* | NS |
| Right kidney wet weight (g/100g of BW) | 0.453 ± 0.016† | 0.521 ± 0.009* | 0.404 ± 0.004 | 0.464 ± 0.009* | NS |
| Urinary protein/creatinine ratio | 9.02 ± 1.63† | 15.12 ± 1.75* | 2.05 ± 0.27 | 3.01 ± 0.39 | P<0.05 |
BW= Mean body weight; ANOVA= Analysis of variance; NS=Not significant Values are mean ± SEM
*p<0.05 versus 0.3% salt diet
†p<0.05 versus Dahl salt-resistant rats fed 0.3% salt diet
Table 1: Hemodynamics, right kidney weight and urinary protein in Dahl salt-sensitive and resistant rats receiving 0.3% salt diet or 4.0% salt diet and vehicle.
In both strains, body weight was less but right kidney wet weight was greater in the rats receiving high salt diet plus vehicle compared with the rats receiving normal salt diet. Mean arterial pressure and urinary protein to creatinine ratio were greater in the rats receiving high salt diet plus vehicle than in the rats receiving normal salt diet in DS strain, although both parameters did not differ significantly between the two groups in DR strain. The effects of salt loading on mean arterial pressure and urinary protein to creatinine ratio differed significantly between both strains (p<0.01 and p<0.05 for strain×salt loading interaction, respectively). On the other hand, pulse rate was lower in the rats receiving high salt diet plus vehicle compared with the rats receiving normal salt diet in both strains. Thus, salt loading aggravated urinary protein excretion concomitantly with the elevation of systemic pressure in DS strain, but not in DR strain.
In both strains, there were no significant differences in body weight and right kidney wet weight between the rats receiving high salt diet plus olmesartan and the rats receiving high salt diet plus vehicle (Table 2). Mean arterial pressure and urinary protein to creatinine ratio were less in the rats receiving high salt diet plus olmesartan compared with the rats receiving high salt diet plus vehicle in DS strain, although these parameters did not differ significantly between the two groups in DR strain. The effects of olmesartan treatment on mean arterial pressure and urinary protein to creatinine ratio significantly differed between the strains (p< 0.05 and p< 0.01 for strain × olmesartan interaction, respectively). Additionally, mean arterial pressure and urinary protein to creatinine ratio did not differ between DS rats receiving high salt plus olmesartan and DR rats receiving high salt plus vehicle. On the other hand, pulse rate did not differ significantly between the rats receiving high salt diet plus olmesartan and the rats receiving high salt diet plus vehicle in either strain. Accordingly, the pubertal treatment with olmesartan prevented the salt-induced increases in mean arterial pressure and urinary protein excretion in DS rats.
| Variables | Dahl salt-sensitive rats | Dahl salt-resistant rats | Strain × olmesartan interaction by two way ANOVA | ||
|---|---|---|---|---|---|
| 4.0% salt diet and vehicle (n=8) | 4.0% salt diet and olmesartan (n=8) | 4.0% salt diet and vehicle (n=8) | 4.0% salt diet and olmesatan (n=8) | P value | |
| BW (g) | 342 ± 9† | 326 ± 10 | 302 ± 7 | 285 ± 0.08 | NS |
| Mean arterial pressure (mmHg) | 147 ± 6† | 117 ± 4* | 112 ± 5 | 108 ± 4 | P<0.05 |
| Pulse rate (beats/min) | 358 ± 16† | 338 ± 12 | 312 ± 8 | 299 ± 12 | NS |
| Right kidney wet weight (g/100g of BW) | 0.521 ± 0.009† | 0.542 ± 0.015 | 0.464 ± 0.009 | 0.482 ± 0.020 | NS |
| Urinary protein/Creatinine ratio | 15.12 ± 1.75† | 3.87 ± 0.65* | 3.01 ± 0.39 | 2.51 ± 0.52 | P<0.01 |
BW= Mean body weight; ANOVA= Analysis of variance; NS=Not significant Values are mean ± SEM
*p< 0.05 versus 4.0% salt diet and vehicle
†p< 0.05 versus Dahl salt-resistant rats fed 4.0% salt diet and vehicle
Table 2: Hemodynamics, right kidney weight and urinary protein in Dahl salt-sensitive and resistant rats receiving 4.0% salt diet and vehicle, and 4.0% salt diet and olmesartan.
Flow-pressure and pressure-GFR relationships in the vasodilated kidneys
A linear relationship was seen between perfusion flow and arterial distending pressure within each individual experiment, with Pearson correlation coefficients (r2) ranging from 0.92 to 0.99. A linear relationship was also seen between arterial distending pressure and GFR within each individual experiment, with r2 ranging from 0.95 to 0.99.
In the rats receiving normal salt diet, there was no significant difference in the gradient of the flow-pressure relationship between both strain (Table 3 and Figure 1). The gradient of the pressure-GFR relationship was less in DS strain than in DR strain, although the X-intercept of the line did not differ significantly between both strains (Figure 2).
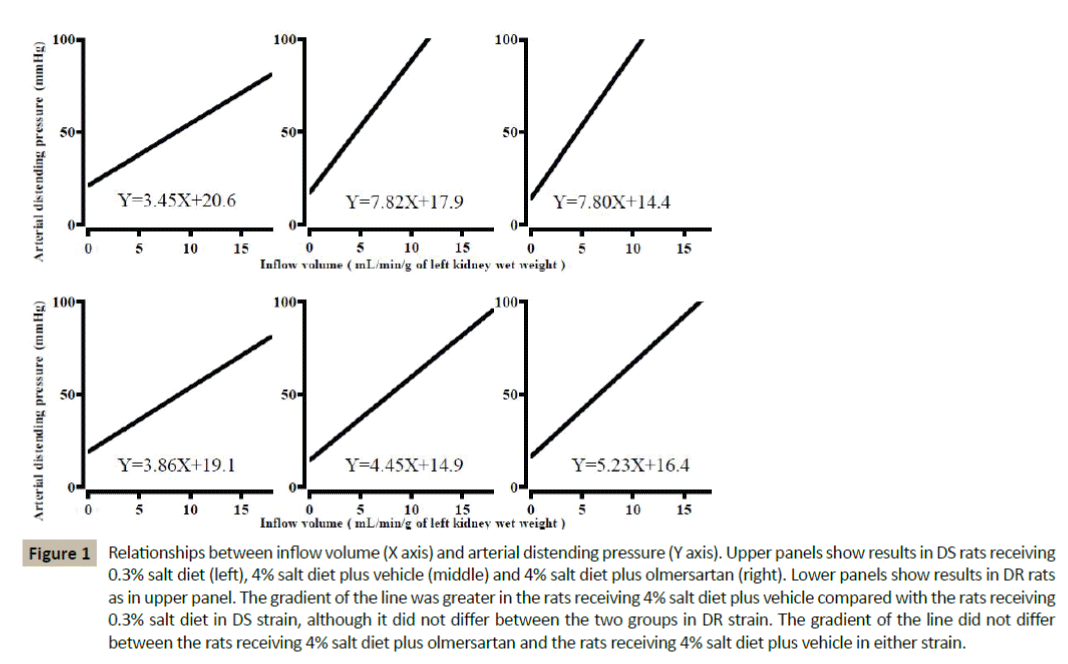
Figure 1: Relationships between inflow volume (X axis) and arterial distending pressure (Y axis). Upper panels show results in DS rats receiving 0.3% salt diet (left), 4% salt diet plus vehicle (middle) and 4% salt diet plus olmersartan (right). Lower panels show results in DR rats as in upper panel. The gradient of the line was greater in the rats receiving 4% salt diet plus vehicle compared with the rats receiving 0.3% salt diet in DS strain, although it did not differ between the two groups in DR strain. The gradient of the line did not differ between the rats receiving 4% salt diet plus olmersartan and the rats receiving 4% salt diet plus vehicle in either strain.
Figure 2: Relationships between arterial distending pressure (X axis) and glomerular filtration rate (GFR, Y axis). Upper panels show results in DS rats receiving 0.3% salt diet (left), 4% salt diet plus vehicle (middle) and 4% salt diet plus olmersartan (right). Lower panels show results in DR rats as in upper panel. In DS strain, the gradient of the line was less in the rats receiving 4% salt diet plus vehicle compared with the rats receiving 0.3% salt diet, although the X-intercept of the line did not differ between the two groups. Contrary, the above parameters were not different between the two groups in DR strain. In DS strain, the gradient of the line was greater in the rats receiving 4% salt diet plus olmersartan compared with the rats receiving 4% salt diet plus vehicle, although the X-intercept of the line did not differ between the two groups. Conversely, the above parameters were not different between the two groups in DR strain.
| Variables | Dahl salt-sensitive rats | Dahl salt-resistant rats | Strain×salt loading interaction by two way ANOVA | ||
|---|---|---|---|---|---|
| 0.3% salt diet (n=8) | 4.0% salt diet and vehicle (n=8) | 0.3% salt diet (n=8) | 4.0% salt diet and vehicle (n=8) | P value | |
| Gradient at F-P relationship (mmHg/mL per min per g of kidney wet weight) | 3.45 ± 0.19 | 7.82 ± 0.35* | 3.86 ± 0.27 | 4.45 ± 0.18 | P<0.05 |
| X-intercept at P-GFR relationship (mmHg) | 35.0 ± 1.2 | 35.8 ± 1.0 | 34.3 ± 1.3 | 33.2 ± 1.3 | ns |
| Gradient at P-GFR relationship (μL/min per g of kidney wet weight per mmHg) | 4.42 ± 0.60† | 3.07 ± 0.21* | 7.09 ± 0.32 | 7.31 ± 0.55 | P<0.05 |
| Interlobular artery | |||||
| Luminal CSA (×102μm2) | 6.13 ± 0.23 | 5.85 ± 0.39* | 6.26 ± 0.38 | 6.07 ± 0.18 | P<0.05 |
| Wall CSA (×102μm2) | 2.36 ± 0.01† | 2.82 ± 0.16* | 2.03 ± 0.09 | 1.96 ± 0.11 | P<0.05 |
| Wall/lumen ratio | 0.388 ± 0.002† | 0.474 ± 0.039* | 0.342 ± 0.010 | 0.333 ± 0.002 | P<0.05 |
| Glomerular tuft area (×102μm2) | |||||
| Superficial glomeruli | 12.09 ± 0.42† | 15.58 ± 0.13* | 11.01 ± 1.01 | 11.02 ± 0.39 | P<0.05 |
| Juxtamedullary glomeruli | 13.47 ± 0.04 | 16.75 ± 0.72* | 12.41 ± 0.68 | 13.28 ± 0.46 | P<0.05 |
| Glomerular sclerosis index | 1.1 ± 0.7 | 15.5 ± 4.2* | 1.0 ± 0.8 | 1.3 ± 0.5 | P<0.05 |
CSA= Cross-sectional area; ANOVA= Analysis of variance; NS= Not significant Values are mean ± SEM
*p<0.05 versus 0.3% salt diet
†p<0.05 versus Dahl salt-resistant rats fed 0.3% salt diet
Table 3: Hemodynamic and histological findings in maximally vasodilated kidneys of Dahl salt-sensitive and resistant rats receiving 0.3% salt diet or 4.0% salt diet and vehicle.
The gradient of the flow-pressure relationship was steeper and the gradient of the pressure-GFR relationship was less in the rats receiving high salt diet plus vehicle compared with the rats receiving normal salt diet in DS strain (Table 3 and Figure 1,2). Contrary, both parameters did not differ significantly between the two groups in DR strain. The effect of salt loading on minimal vascular change and glomerular filtration capacity against pressure differed significantly between both strains (p<0.05 and p<0.05 for strain×salt loading interaction, respectively). On the other hand, the X-intercept of the pressure-GFR relationship did not differ significantly between the two groups in either strain.
In DS strain, the gradient of the pressure-GFR relationship was greater in the rats receiving high salt plus olmesartan than in the rats receiving high salt plus vehicle, although the gradient of the flow-pressure relationship and the X-intercept of the pressure-GFR relationship did not differ between the two groups (Table 4 and Figure 1,2). Contrary, the above three indices did not differ between the two groups in DR strain. The effect of olmesartan treatment on glomerular filtration capacity against pressure differed significantly between both strains (p<0.05 for strain×olmesartan interaction). However, glomerular filtration capacity against pressure remained to be less in DS rats receiving high salt plus olmesartan compared with DR rats receiving high salt plus vehicle (p<0.05).
| Variables | Dahl salt-sensitive rats | Dahl salt-resistant rats | Strain×olmesartan interaction by two way ANOVA | ||
|---|---|---|---|---|---|
| 4.0 % salt diet and vehicle (n=8) | 4.0 % salt diet and olmesartan (n=8) | 4.0 % salt diet and vehicle (n=8) | 4.0 % salt diet and olmesatan (n=8) | P value | |
| Gradient at F-P relationship (mmHg/mL per min per g of kidney wet weight) | 7.82 ± 0.35† | 7.80 ± 0.49 | 4.45 ± 0.17 | 5.23 ± 0.55 | NS |
| X-intercept at P-GFR relationship (mmHg) | 35.8 ± 1.0 | 34.6 ± 0.7 | 33.2 ± 1.3 | 32.3 ± 1.0 | NS |
| Gradient at P-GFR relationship (µL/min per g of kidney wet weight per mmHg) | 3.07 ± 0.21† | 4.09 ± 0.29* | 7.31 ± 0.55 | 7.54 ± 0.78 | P<0.05 |
| Interlobular artery | |||||
| Luminal CSA (×102μm2) | 5.85 ± 0.39† | 5.99 ± 0.17 | 6.07 ± 0.18 | 6.12 ± 0.23 | |
| Wall CSA (×102μm2) | 2.82 ± 0.16† | 2.36 ± 0.08* | 1.96 ± 0.11 | 1.95 ± 0.13 | P<0.05 |
| Wall/lumen ratio | 0.474 ± 0.039† | 0.391 ± 0.005* | 0.334 ± 0.002 | 0.326 ± 0.011 | P<0.05 |
| Glomerular tuft area (×102μm2) | |||||
| Superficial glomeruli | 15.58 ± 0.13† | 14.11 ± 0.41 | 11.02 ± 0.39 | 11.61 ± 0.22 | NS |
| Juxtamedullary glomeruli | 16.75 ± 0.72† | 16.17 ± 0.27 | 13.28 ± 0.46 | 13.65 ± 0.22 | NS |
| Glomerular sclerosis index | 15.5 ± 4.2† | 12.8 ± 6.1 | 1.3 ± 0.5 | 1.2 ± 0.9 | NS |
CSA= Cross-sectional area; ANOVA= Analysis of variance; NS= Not significant Values are mean ± SEM
*p<0.05 versus 4.0% salt diet and vehicle
†p<0.05 versus Dahl salt-resistant rats fed 4.0% salt diet and vehicle
Table 4: Hemodynamic and histological findings in maximally vasodilated kidneys of Dahl salt-resistant and resistant rats receiving 4.0% salt diet and vehicle, and 4.0% salt diet and olmesartan.
Histological findings in the vasodilated kidneys
In the rats receiving normal salt diet, the wall cross-sectional area of the interlobular arteries (one of the preglomerular resistance vessels) was significantly greater in DS strain than in DR strain, although the internal luminal area of these vessels did not differ significantly between both strains (Table 3). Consequently, the wall-to-lumen ratio was greater in DS strain compared with DR strain. Glomerular tuft area was also greater in DS strain compared with DR strain especially at superficial glomeruli, but glomerular sclerosis was not apparent in either strain.
The wall cross-sectional area was greater but the internal luminal area was less in the rats receiving high salt diet plus vehicle compared with the rats receiving normal salt diet in DS strain (Table 3). Consequently, the wall-to-lumen ratio was greater in the rats receiving high salt diet plus vehicle than in the rats receiving normal salt diet. Glomerular tuft area and glomerular sclerosis index was also greater in the rats receiving high salt diet plus vehicle than in the rats receiving normal salt diet in DS strain. Contrary, the above morphological parameters for the interlobular arteries and glomeruli did not differ between the two groups in DR strain. The effects of salt loading on these morphological parameters were significantly different between both strains (p<0.05 for strain×salt loading interaction in each parameter).
In DS strain, the wall cross-sectional area of the interlobular arteries was less in the rats receiving high salt diet plus olmesartan than in the rats receiving high salt diet plus vehicle, although the internal luminal area did not differ significantly between the two groups (Table 4). As a result, the wall-to-lumen ratio was less in the rats receiving high salt diet plus olmesartan compared with the rats receiving high salt diet plus vehicle in DS strain. In contrast, the above morphological parameters for the interlobular arteries did not differ between the two groups in DR strain. However, the wall-to-lumen ratio remained greater in DS rats receiving high salt diet plus olmesartan compared with DR rats receiving high salt diet plus vehicle (p< 0.05). On the other hand, glomerular tuft area and glomerular sclerosis index did not differ between the two groups in either strain.
Discussion
To the best of our knowledge, this study was the first to ascertain the effects of salt loading on renal vascular structures and their potential to contribute to the development of hypertension in DS rats. We also demonstrated ARB administered briefly prior to salt loading partially ameliorated these effects in DS rats. The major findings of this study are as follows. Firstly, at the pre-hypertensive stage in DS rats fed normal salt diet, the decrease in glomerular filtration capacity against pressure already occurred with both minimal renal vascular resistance and preglomerular: postglomerular vascular resistance ratio being normal, a finding consistent with our previous study [14]. This renal hemodynamic alteration was characterized histologically by hypertrophic vascular remodeling without luminal changes at the interlobular arteries and glomerular enlargement. Secondly, in DS rats, salt loading increased minimal renal vascular resistance without changes in the pre-glomerular: post-glomerular vascular resistance ratio. That is that, in DS rats fed high salt diet, vascular narrowing appeared to have occurred in both preglomerular and postglomerular resistance vessels to the similar extent concomitantly with the development of hypertension and aggravation of urinary protein excretion. Additionally, salt loading worsened the reduction in glomerular filtration capacity against pressure further in DS rats. Morphologically, at the interlobular arteries of DS rats, salt loading progressed vascular hypertrophy and narrowed vascular lumen (i.e., inward vascular remodeling), leading to the elevation in wall-to-lumen ratio. Salt loading also accelerated glomerular enlargement with the occurrence of glomerular sclerosis in DS rats. Contrary, the effects of salt loading on the above-mentioned parameters were not observed in DR rats. Thirdly, the pubertal treatment with olmesartan prevented the development of hypertension and aggravation of urinary protein excretion following salt loading in DS rats. Simultaneously, in DS rats fed high salt diet, the treatment with olmesartan prevented the worsening of reduction in glomerular filtration capacity, although it did not affect minimal renal vascular resistance and preglomerular: postglomerular vascular resistance ratio. Morphologically, the olmesartan treatment also attenuated vascular hypertrophy and decreased wall-to-lumen ratio in the interlobular arteries of DS rats fed high salt diet. The above-mentioned effects of olmesartan under salt loading were specific to DS rats.
Implications of renal structural properties following salt loading in DS rats
Taking into account the amplification of responses to vasoactive stimuli in vascular structural remodeling [11-13,23-25] the following sequence of events may ensue following salt loading in DS rats even if the neurohumoral influences on the kidneys remain to be unchanged. In the present study, vascular hypertrophic remodeling without luminal changes in the preglomerular resistance vessel and reduction in glomerular filtration capacity against pressure occurred prior to the development of hypertension in DS rats on normal salt diet. Such renal vascular structural properties at the pre-hypertensive stage could serve to increase renal vascular resistance and reduce renal excretory capability in DS rats, especially on high salt diet. Therefore, in DS rats, salt loading could increase intravascular pressure and volume, possibly leading to the development of hypertension. Furthermore, salt-induced increases in intravascular pressure and volume could be amplified because of the concomitant occurrences of vascular narrowing at renal resistance vessels and worsening of the reduction in glomerular filtration capacity in DS rats. Additionally, in the kidneys of DS rats, salt-induced elevation in systemic blood pressure could increase intraglomerular pressure because the degree of pressure drop from the aorta to Bowman’s capsule (i.e. preglomerular: postglomerular vascular resistance ratio) remained unaffected by salt loading. This intraglomerular hypertension could destroy the glomerular architecture, leading to the aggravations of impairment in renal excretory capability and glomerular sclerosis in DS rats. These changes are consistent with the findings obtained in DS rats in vivo [4,7-9]. Of note is that such intrarenal hemodynamic changes could be also confounded or modified by the alterations of other factors including intrarenal autoregulation mechanism, vascular reactivity and endothelial function [26-29].
Effects of a brief administration of ARB during pubertal period on salt-induced renal vascular structural changes in DS rats
The short-term treatment of SHR with RAS inhibitors at the pre-hypertensive or early hypertensive period can produce a sustained reduction of blood pressure even after discontinuation of the treatment [15-17]. This primary prevention of hypertension using antihypertensive drugs is specific to RAS inhibitors, because Ca antagonists or vasodilators do not have such effects [16]. The primary prevention of hypertension using RAS inhibitors in animals is also validated clinically in human prehypertension by the TROPHY study [30]. In the present study, the brief treatment of DS rats with olmesartan at pubertal period suppressed salt-induced increases in both blood pressure and proteinuria, a finding consistent with a previous study [18]. Thus, in hereditary hypertensive animals, the transient inhibition of RAS at pre-hypertensive or early hypertensive stage results in prevention of the later development of hypertension and hypertensive renal damages, regardless of the susceptibility of animals to blood-pressure-raising effect of salt [15-18]. Additionally, the treatment of DS rats with olmesartan ameliorated the salt-induced decline in glomerular filtration capacity and retarded the vascular hypertrophy with the reduction in wall to lumen ratio. Taking into account the possible role of structural properties in renal hemodynamics, the effects of olmesartan on renal vascular structures could enhance renal excretory capability and decrease the vascular contractile response to vasoactive stimuli in renal resistance vessels in DS rats, thereby attenuating the salt-induced hypertension. The subsequent reduction in systemic pressure could also decrease inraglomerular pressure, leading to the reduction in urinary protein excretion. Thus, the effects of olmesartan on the saltinduced renal vascular structural alterations could contribute possibly to the antihypertensive and renoprotective actions of pubertal ARB treatment in DS rats, as shown in SHR by Smallegange C et al [17].
The possible mechanism contributing to the renal structural responses to salt loading in DS rats
It is well known that cardiovascular system can remodel its own structure to adapt to the prevailing conditions of pressure or flow on the vascular beds so that its wall tension or hemodynamic shear rate can remain constant [23,24]. It is therefore theoretically conceivable in DS rat that the renal vasculature could be remodelled in response to salt loading as follows. In DS rats, salt loading could increase intravascular pressure and volume because of renal structural properties at pre-hypertensive stage. Thereafter, the resultant pressure and volume overload in the renal vasculature could affect the growth of the renal vasculature and change the renal vascular architecture. That is, when pressure increases, the lumen size is reduced by either an increase in muscular mass or rearrangement of cellular and non-cellular elements (i.e. hypertrophic or eutrophic inward remodelling). In contrast, increased flow induces outward hypertrophic remodelling resulting in luminal widening. Of course, salt-induced alterations in renal vasculature tree could also be modified by non-hemodynamic factors such as growth factors including angiotensin II [23,24]. Indeed, the inhibition of angiotensin II action with olmesartan prevented the salt-induced alterations in renal vascular structures in DS rats. However, it cannot be deduced from the present study whether the effects of olmesartan on renal structural responses to salt loading could be attributed to the inhibition on vascular hypertrophic action of angiotensin II, reduction in blood pressure secondary to the inhibition on hemodynamic action of angiotensin II or both in DS rats.
Methodological Considerations
Methodological aspects of the present study deserve comment. Firstly, the dose of olmesartan used in the present study (10 mg/ kg/day) is effective in lowering blood pressure in hypertensive rats [31,32]. The time and interval of treatment with RAS inhibitors (3 to 4 weeks of age and 2 weeks, respectively) is suitable for the long-lasting prevention of hypertension in SHR [15,17]. Therefore, our study design is acceptable in terms of administration of olmesartan. Secondly, renal vascular structure was assessed hemodynamically using an in vitro, maximally dilated whole-kidney perfusion technique, as in previous studies [14,20,21]. In contrast with histological analysis, this physiological technique is accurate and sensitive enough to detect subtle changes in lumen diameter in resistance vessels, as follows. It can determine vascular luminal changes at the whole renal resistance vessels (i.e., pre- and post-glomerular vasculature). It can also determine whether the luminal changes are confined to either the pre- or post-glomerular circulation. However, we cannot determine whether luminal changes at the preglomerular circulation occurred in the afferent arteriole, larger upstream vessels or both, or whether luminal changes were located in the cortical or medullary vessels.
Although limited for these reasons, it clearly demonstrated that inward vascular hypertrophy without changes in the preglomerular: postglomerular vascular resistance ratio in the renal resistance vessels and worsening of the impairment in glomerular filtration capacity could occur following salt loading in DS rats. In DS rats, the brief treatment with olmesartan during pubertal period prior to salt loading could prevent the later development of salt-induced hypertension possibly via the inhibition of salt-induced alterations in renal vascular structures.
References
- Fujita T, Henry WL, Bartter FC, Lake CR, Delea CS (1980) Factors influencing blood pressure in salt-sensitive patients with hypertension. Am J Med69:334-344.
- Campese VM, Parise M, Karubian F, Bigazzi R (1991)Abnormal renal hemodynamics in black salt-sensitive patients with hypertension. Hypertension18:805-812.
- Kimura G, Brenner BM (1997) Implications of the linear pressure-natriuresis relationship and importance of sodium sensitivity in hypertension. J Hypertens15:1055-1061.
- Azar S, Limas C, Iwai J, Weller D (1979) Single-nephron dynamics during high sodium intake and early hypertension in Dahl rats. Jpn Heart J20 (suppl I):138-140.
- Dworkin LD, Hostetter TH, Rennke HG, Brenner BM (1984) Hemodynamic basis for glomerular injury in rats with desoxycorticosterone-salt hypertension. J Clin Invest73:1448-1461.
- Anderson S, Meyer TW, Rennke HG, Brenner BM (1985) Control of glomerular hypertension limits glomerular injury in rats with reduced renal mass. J Clin Invest76:612-619.
- Sterzel RB, Luft FC, Gao Y, Schnermann J, Briggs JP, et al. (1988) Renal disease and the development of hypertension in salt-sensitive Dahl rats. Kidney Int33:1119-1129.
- Raij L, Azar S, Keane W (1984)Mesangial immune injury, hypertension, and progressive glomerular damage in Dahl rats. Kidney Int26:137-143.
- Rapp JP (1982) Dahl salt-susceptible and salt-resistant rats: a review. Hypertension4:753-763.
- Morimoto A, Uzu T, Fujii T, Nishimura M, Kuroda S, et al. (1997) Sodium sensitivity and cardiovascular events in patients with essential hypertension. Lancet350:1734-1737.
- Folkow B (1982) Physiological aspects of primary hypertension. Physiol Rev62:347-504.
- Korner PI, Angus JA (1992) Structural determinants of vascular resistance properties in hypertension: haemodynamic and model analysis. J Vasc Res29:293-312.
- Folkow B (1995) Hypertensive structural changes in systemic precapillary resistance vessels: how important are they for in vivo haemodynamics? J Hypertens13:1546-1559.
- Tomoda F, Takata M, Kinuno H, Tomita S, Yasumoto K, et al. (2000) Renal structural properties in prehypertensive Dahl salt-sensitive rats. Hypertension36:68-72.
- Harrap SB, Merwe WM, Griffin SA, Macpherson F, Lever AF (1990) Brief angiotensin converting enzyme inhibitor treatment in young spontaneously hypertensive rats reduces blood pressure long-term. Hypertension16:603-614.
- Nakaya H, Sasamura H, Hayashi M, Saruta T (2001) Temporary treatment of prepubescent rats with angiotensin inhibitors suppresses the development of hypertensive nephrosclerosis. J Am SocNephrol12:659-666.
- Smallegange C, Hale TM, Bushfield TL, Adams MA (2004) Persistent lowering of pressure by transplanting kidneys from adult spontaneously hypertensive rats treated with brief antihypertensive therapy. Hypertension44:89-94.
- Nakaya H, Sasamura H, Mifune M, Shimizu-Hirota R, Kuroda M, et al. (2002) Prepubertal treatment with angiotensin receptor blocker causes partial attenuation of hypertension and renal damage in adult Dahl salt-sensitive rats. Nephron91:710-718.
- Schwab SJ, Christensen RL, Dougherty K, Klahr S (1987) Quantitation of proteinuria by the use of protein-to-creatinine ratios in single urine samples. Arch Intern Medicine147:943-944.
- Koike T, Tomoda F, Kinuno H, Inoue H, Takata M (2005) Abnormal renal structural alterations during the development of diabetes mellitus in Otsuka Long-Evans Tokushima Fatty rats. ActaPhysiolScand184:73-81.
- Kinuno H, Tomoda F, Koike T, Takata M, Inoue H (2005) Effects of uninephrectomy on renal structural properties in spontaneously hypertensive rats. ClinExpPharmacolPhysiol32:173-178.
- Davidson WD, Sackner MA (1963) Simplification of the anthrone method for the determination of inulin in clearance studies. J Lab Clin Med62:351-356.
- Mulvany MJ (1999)Vascular remodeling of resistance vessels: can we define this? Cardiovasc Res41:9-13.
- Gibbons GH, Dzau VJ (1994)The emerging concept of vascular remodeling. N Engl J Med330:1431-1438.
- Simchon S, Manger WM, Brown TW (1991) Dual hemodynamic mechanisms for salt-induced hypertension in Dahl salt-sensitive rats. Hypertension17:1063-1071.
- Karlsen FM, Andersen CB, Leyssac PP, Holstein-Rathlou NH (1997) Dynamic autoregulation and renal injury in Dahl rats. Hypertension30:975-983.
- Simchon S, Manger WM, Shi GS, Brensilver J (1992) Impaired renal vascular reactivity in prehypertensive Dahl salt-sensitive rats. Hypertension20:524-532.
- Chen PY, Sanders PW (1991) L-Arginine abrogates salt-sensitive hypertension in Dahl/Rapp rats. J Clin Invest88:1559-1567.
- Hayakawa H, Hirata Y, Suzuki E, Sugimoto T, Matsuoka H, et al. (1993) Mechanisms for altered endothelium-dependentvasorelaxation in isolated kidneys from experimental hypertensive rats. Am J Physiol. 264:H1535-H1541.
- Julius S, Nesbitt SD, Egan BM, Weber MA, Michelson EL, et al. (2006) Feasibility of treating prehypertension with an angiotensin-receptor blocker. N Engl J Med354:1685-1697.
- Xu HL, Yoshida K, Wu XM, Kohzuki M (2001) Effects of CS-866, an angiotensin II receptor antagonist, in 5/6 nephrectomized spontaneously hypertensive rats.Jpn J Nephrol43:580-588.
- Kawasaki H, Inaizumi K, Nakamura A, Hobara N, Kurosaki Y (2003) Chronic angiotensin II inhibition increases levels of calcitonin gene-related peptide mRNA of the dorsal root ganglia in spontaneously hypertensive rats.Hypertens Res26:257-263.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences