Insights in Blood Pressure
Larry H Bernstein
Larry H Bernstein*
Retired Pathologist, Bridgeport Hospital, Yale New Haven Health, United States
- Corresponding Author:
- Larry H Bernstein
Retired Pathologist, Bridgeport Hospital
Yale New Haven Health, US
Tel: 2032618671
E-mail: larry.bernstein@gmail.com
Received Date: September 15, 2015, Accepted Date: September 18, 2015, Published Date: September 25, 2015
Citation: Bernstein LH. Insights in Blood Pressure. Insights Blood Press 2015, 1:1.
A review of the physiology of blood pressure and the pathogenesis of hypertension (or of hypotension) can be a very large undertaking, considering the vast literature only in the last half century. The main considerations to be taken into account are: disease comorbidities, the heart, kidney and arterial vasculature; dietary factors; major life stresses; and endocrine and neuroendocrine considerations. These will be discussed, not necessarily in the order stated.
The physiology of blood pressure is probably familiar to most readers of this publication. What has been well known is the association between elevated blood pressure and obesity, diabetes, and the prescription for congestive heart disease (myocardial hypertrophy). The importance of stress in generating hypertension long term is more likely to be overlooked because it is so common, and yet in the 19th century England it was observed that workers had greater risk for cardiovascular risk than “bosses”.
Normal arterial blood pressures are a systolic pressure 90-119 mmHg and a diastolic pressure 60-79 mmHg. An arterial pressure in the pre-hypertensive range is ≥ 120/80 mmHg. Mean arterial pressure is not usually measured in people. The diastolic value has been emphasized more in assessing hypertension, but that is not the case in the elderly. Elevations in systolic pressure ("systolic hypertension") are associated with increased incidence of coronary and cerebrovascular disease (e.g., stroke). We recognize that both systolic and diastolic pressure values are important [1]. According to US. national guidelines (JNC 7 Report and JNC 8 Report), the following represents different stages of hypertension (Table 1)
| Classification | Systolic (mmHg) | Diastolic (mmHg) |
|---|---|---|
| Normal | <120 | <80 |
| Pre-Hypertension | 120-139 | 80-89 |
| Stage 1 | 140-159 | 90-99 |
| Stage 2 | >160 | >100 |
Table 1 Factors Influencing LV Geometry in Hypertensive Patients Pressure load: severity, duration, rapidity of onset Volume load Demographic factors: age, race/ethnicity, gender Concomitant medical conditions: coronary artery disease, diabetes mellitus, obesity, valvular heart disease Neurohormonal milieu Alterations of the extracellular matrix Genetic factors.
In 90-95% of patients presenting with hypertension, the cause is unknown. This condition is called primary (or essential) hypertension. In the remaining 5-10% of hypertensive patients the hypertension results secondarily from renal disease, endocrine disorders, or other identifiable causes. This form of hypertension is called secondary hypertension.
Regardless of the origin of hypertension, the actual increase in arterial blood pressure is caused by either an increase in systemic vascular resistance (SVR), determined by the vascular tone, or by an increase in cardiac output (CO). It is then necessary to understand the mechanisms that regulate both SVR and CO to understand elevated arterial blood pressure.
The pathogenesis of essential hypertension is multifactorial and highly complex. The factors that modulate blood pressure (BP) include the following:
• Humoral mediators
• Vascular reactivity
• Circulating blood volume
• Vascular caliber
• Blood viscosity
• Cardiac output
• Blood vessel elasticity
• Neural stimulation
Over the course of its natural history, essential hypertension progresses from occasional to established hypertension [2]. After a long, invariable, asymptomatic period, persistent hypertension develops into complicated hypertension, exhibiting damage to the aorta and small arteries, heart, kidneys, retina, and central nervous system is evident.
The progression of essential hypertension begins with increased cardiac output in persons aged 10-30 years and this advances in persons aged 20-40 years to increased peripheral resistance, or to established hypertension in persons aged 30-50 years, and finally to complicated hypertension in persons aged 40-60 years.
Arterial BP is a product of cardiac output and peripheral vascular resistance. The factors affecting cardiac output include sodium retention, renal function, and mineralocorticoids, which have an inotropic effect due to extracellular fluid volume and causes increased heart rate and contractility. The regulation of circulating blood volume by renal salt and water handling is a phenomenon that plays a particularly important role in saltsensitive hypertension.
Peripheral vascular resistance is highly dependent upon the sympathetic nervous system, humoral factors, and local auto regulation. The sympathetic nervous system produces its effects via the vasoconstriction (alpha effect) or vasodilation (beta effect). Studies with bilateral radiofrequency renal nerve ablation have shown a significant reduction of blood pressure in drug-resistant patients, and reductions in blood pressure have shown bilateral carotid artery stimulation in the same populations, which confirm the role of the sympathetic nervous system in the pathogenesis of hypertension.
The humoral actions on peripheral resistance are influenced by vasoconstrictors (e.g., angiotensin, catecholamine) and vasodilators (e.g., prostaglandins, kinins). The vasoreactivity of the vascular bed mediates changes of hypertension, influenced by the activity of these vasoactive factors, reactivity of the smooth muscle cells, and structural changes in the vessel wall and vessel caliber, expressed by a lumen-to-wall ratio. Blood viscosity, vascular wall shear conditions (rate and stress), and blood flow velocity (mean and pulsatile components) play an added role in the regulation of BP in humans by vascular and endothelial function.
Auto regulation of BP occurs by way of intravascular volume contraction and expansion regulated by the kidney, as well as via transfer of trans capillary fluid. Salt and water balance is achieved at heightened systemic pressure by a mechanism of pressure natriuresis, proposed by Guyton. Interactions between cardiac output and peripheral resistance are auto regulated to maintain a set BP in an individual. For example, constriction of the arterioles elevates arterial pressure by increasing total peripheral resistance, whereas venular constriction leads to redistribution of the peripheral intravascular volume to the central circulation, thereby increasing preload and cardiac output.
The vascular endothelium is considered to be a vital organ, in which synthesis of various vasodilating and constricting mediators occurs. The interaction of autocrine and paracrine factors takes place in the vascular endothelium, leading to growth and remodeling of the vessel wall and to the hemodynamic regulation of BP.
Numerous hormonal, humoral vasoactive, and growth and regulating peptides are produced in the vascular endothelium. These mediators include angiotensin II, bradykinin, endothelin, nitric oxide, and several other growth factors. Endothelin is a potent vasoconstrictor and growth factor that likely plays a major role in the pathogenesis of hypertension. Angiotensin II is a potent vasoconstrictor synthesized from angiotensin I with the help of an angiotensin-converting enzyme (ACE).
Another vasoactive substance manufactured in the endothelium is nitric oxide. Nitric oxide is an extremely potent vasodilator that influences local auto regulation and other vital organ functions. Additionally, several growth factors manufactured in the vascular endothelium include platelet-derived growth factor, fibroblast growth factor, insulin growth factor, and many others.
Patients who develop hypertension are sensitive to vasoconstrictive stimuli. Vascular remodeling occurs over the years as hypertension evolves, thereby maintaining increased vascular resistance irrespective of the initial hemodynamic pattern. Changes in vascular wall thickness affect the amplification of peripheral vascular resistance in hypertensive patients and result in the reflection of waves back to the aorta, increasing systolic BP.
One form of essential hypertension, termed high-output hypertension, results from decreased peripheral vascular resistance and concomitant cardiac stimulation by adrenergic hyperactivity and altered calcium homeostasis. A second mechanism manifests with normal or reduced cardiac output and elevated systemic vascular resistance (SVR) due to increased vasoreactivity. Another (and overlapping) mechanism is increased salt and water reabsorption (salt sensitivity) by the kidney, which increases circulating blood volume.
Chronic hypertension leads to increased arterial stiffness, increased systolic blood pressure (BP), and widened pulse pressures. These factors decrease coronary perfusion pressures, increase myocardial oxygen consumption, and lead to left ventricular hypertrophy (LVH). Consequently, the myocardium undergoes structural changes in response to increased afterload. Cardiac myocytes allow the heart to pump more strongly against the elevated pressure. However, the contractile function of the left ventricle remains normal until later stages. Eventually, LVH lessens the chamber lumen, limiting diastolic filling and stroke volume. The left ventricular diastolic function is markedly compromised in long-standing hypertension. The mechanisms of diastolic dysfunction apparently include an aberration in the passive relaxation of the left ventricle during diastole.
It was recently reported that one in three people have hypertension. The result of a long term study resulted in a recommendation to lower the blood pressure limit from 140 to 120 mmHg systolic, and from 90 to 60 mm Hg [3]. The diastolic blood pressure that measures the pressure at heart rest was of concern because of the risk of bringing the pressure too low. Targets released last year advised doctors to loosen treatment targets for patients with high blood pressure. Most patients over age 60 were advised to shoot for a goal of 150/90.
The Agency for Healthcare Research and Quality (A Systematic Evidence Review for the US. Preventive Services Task Force, Evidence Syntheses, No. 121, Report No.: 13-05194-EF-1): Agency for Healthcare Research and Quality (US)) conducted a dual independent review of 19,309 abstracts and 1,171 full-text articles against a priori inclusion and exclusion criteria.
Two investigators also independently critically appraised each included article using criteria defined by the USPSTF and supplemented with criteria from the Quality Assessment of Studies of Diagnostic Accuracy II, the Quality in Prognosis Studies tool, and the Newcastle-Ottawa Scale for diagnostic accuracy (KQs 2 and 3), prognostic (KQ 3), and observational KQs 4 and 5) studies, respectively. We resolved discrepancies through discussion and consultation with a third reviewer, when necessary. We included only fair- or good-quality studies.
For KQs 1 and 5, the results were qualitatively summarized because of the small number of included studies. For KQ 2, they calculated the diagnostic accuracy of office-based BP measurement (OBPM) devices and protocols using the result from the most commonly recommended device (i.e., manual mercury sphygmomanometer) or protocol component (e.g., no caffeine) as the reference standard. They qualitatively summarized the results.
For the prognosis component of KQ3, and grouped outcomes into the categories of cardiovascular (CV), stroke, and cardiac events, they combined fatal and nonfatal events within these outcome categories. Risk was consistently expressed as a hazard ratio per increment in BP measurement across all included studies. Risk results for CV outcomes by BP measurement method at baseline were visualized in forest plots of hazard ratios. For diagnostic accuracy calculations, we used the BP measurement method that best predicted CV outcomes (i.e., ambulatory BP monitoring [ABPM]) as the reference standard. Qualitative evaluations of how patient or study characteristics influenced diagnostic accuracy were as follows:
For KQ 4, the pooled incidence rates for the overall populations in included studies to generate a weighted mean incidence at various rescreening intervals, were categorized into 1, 2, 3, 4, and 5 years. They qualitatively examined direct evidence from subgroup results reported within studies to address the influence of patient characteristics.
One randomized, controlled trial (39 clusters; n=140,642) of a Canadian BP screening program that targeted adults age 65 years or older reported 3.02 fewer annual hospital admissions for cardiovascular disease per 1,000 persons in the intervention group compared with the no screening group. When the trial data were analyzed by number of unique persons with hospital admissions, there was a significant relative reduction only in the individual outcome of acute myocardial infarction (rate ratio, 0.89 [95% CI, 0.79 to 0.99]; p=0.03).
The likelihood of misdiagnosis of hypertension based only on screening measurement is greater as measurements approach the threshold for a diagnosis of hypertension. They did not qualitatively detect any associations between reported race/ ethnicity, sex, or smoking. Hypertension incidence increased as much as two- to four-fold between a younger (ages 18 to 40/45 years) and older (ages 40/45 to 60/65) age group, respectively.
Within-study hypertension incidence consistently tripled when comparing participants with initial optimal versus normal BP, and was approximately doubled in those with initial normal versus high-normal BP. Incidence was generally higher in men than women, especially men in younger populations. While incidence was also two-fold higher in overweight persons and three-fold higher in obese persons compared with those of normal weight, it was not increased in smokers compared with nonsmokers or former smokers. African Americans had a consistently higher incidence of hypertension at rescreening than white participants. A review of 49 research studies was done for the Agency for Healthcare Research and Quality (AHRQ), a Federal research agency, to understand the benefits of self-measuring blood pressure. The report was reviewed by clinicians, researchers, experts, and the public. You can read the report at www. effectivehealthcare.ahrq.gov/selfmeasuredbp.cfm .
The relationship of hypertension to the prediction of myocardial infarction and to stroke is well established, and is concordant with lipidemic disorders of high LDL and low HDL, as well as LDL subtypes https://www.ncbi.nlm.nih.gov/pubmedhealth/ PMHT0024199/.
ABPM (24-hr, daytime, or nighttime) is a better predictor of long-term CV outcomes than OBPM (usually manual sphygmomanometer) and should be considered the reference standard for evaluating noninvasive BP measurements. A small body of evidence suggests that HBPM can serve as a similar predictor of outcomes. Studies of rescreening intervals at up to 6 years found a higher incidence of hypertension overall and at shorter intervals for persons with BP in the high-normal range, older adults, persons with an above normal BMI, and African Americans. These studies showed much lower incidence at longer rescreening intervals up to 6 years in persons without these risk factors.
Calcium antagonists (calcium channel blockers [CCBs]) have a number of roles, and they are certainly some superb blood pressure-lowering agents [4]. But there are differences with the calcium antagonists with regard to heart rate, sympathetic nervous system activation, and cardiovascular events related to diabetes, angina, and kidney disease progression.
Heart rates are clearly predictive of mortality, and it's clear that once you get heart rates of 84 beats/min or higher, mortality from coronary heart disease goes up dramatically. When you look at the beta-blocker trials in relationship to cardiovascular mortality, the agents that reduce heart rate, even among the beta-blockers, are shown to have a reduction in cardiovascular mortality-not the beta-blockers with intrinsic and sympathomimetic activity.
Hypertension is a complex quantitative trait under polygenic control. The identification of genes responsible for high blood pressure provides a mechanistic classification of the common phenotype and guide therapy tailored to the underlying primary abnormality [5]. Experimental studies have identified several quantitative trait loci for blood pressure and other cardiovascular phenotypes. Human studies have focused on the rare Mendelian forms of human hypertension or candidate gene studies.
A direct, continuous, and independent relation between blood pressure and the incidence of various cardiovascular events, such as stroke and myocardial infarction, is now well accepted. The increase in risk can be attributed to structural and functional changes in target organs. Central to many of these pathophysiologic processes is the renin-angiotensin system (RAS), specifically, angiotensin II. Binding of angiotensin II to angiotensin II type-1 (AT1) receptors produces acute vasoconstriction, leading to an increase in blood pressure [6].
AT1 receptor activation also contributes independently to chronic disease pathology by promoting vascular growth and proliferation, and endothelial dysfunction. These negative consequences of angiotensin II are partly counteracted by angiotensin II type- 2 (AT2) receptor stimulation, which has favorable effects on tissue growth and repair processes. Thus, the use of selective AT1 receptor antagonists in the treatment of hypertension has a 2-fold rationale: (1) selective AT1 receptor blockade targets the final common pathway for all major detrimental cardiovascular actions of angiotensin II, and (2) circulating angiotensin II levels (which increase during AT1 receptor antagonist treatment) will be free to act only at unopposed AT2 receptors, potentially providing additional end-organ protection. Angiotensin-converting enzyme (ACE) inhibitors interrupt the RAS by preventing the conversion of angiotensin I to angiotensin II.
Diabetes mellitus is commonly associated with systolic/diastolic hypertension, an association that is independent of age and obesity. Much evidence indicates that the link between diabetes and essential hypertension is hyperinsulinemia. Hypertensive patients, whether obese or of normal body weight, compared with age- and weight-matched normotensive control subjects, have a heightened plasma insulin response to a glucose challenge. The insulin resistance of essential hypertension has been shown to be located in peripheral tissues (muscle) correlates directly with the severity of hypertension.
The reasons for the association of insulin resistance and essential hypertension can be sought in at least four general types of mechanisms [7]: Na+ retention, sympathetic nervous system overactivity, disturbed membrane ion transport, and proliferation of vascular smooth muscle cells. Calorie restriction and regular physical exercise can improve tissue sensitivity to insulin; evidence also indicates that these maneuvers can also lower blood pressure in both normotensive and hypertensive individuals. Insulin resistance and hyperinsulinemia are also associated with an atherogenic plasma lipid profile. Reducing lipid and apolipoproteins from the VLDL particle leads to an increased formation of intermediate-density and low-density lipoproteins, both of which are atherogenic.
Atherogenicity of insulin is independent of its effects on blood pressure and plasma lipids as it enhances cholesterol transport into arteriolar smooth muscle cells and increases endogenous lipid synthesis by these cells. Insulin also stimulates the proliferation of arteriolar smooth muscle cells, augments collagen synthesis in the vascular wall, increases the formation of and decreases the regression of lipid plaques, and stimulates the production of various growth factors. In summary, insulin resistance appears to be a syndrome that is associated with a clustering of metabolic disorders, including non-insulin-dependent diabetes mellitus, obesity, hypertension, lipid abnormalities, and atherosclerotic cardiovascular disease.
Insulin resistance, characterized by hyperinsulinemia and normal or elevated serum glucose, is an established precursor to diabetes and cardiovascular disease. A retrospective cohort study used data from the NHANES?3 (1988–1994) survey with mortality follow?up through December 31, 2006 [8]. Participants included 5153 subjects, 40 to 74 years of age with fasting glucose ≥ 70 mg/dL, without diabetes by history or laboratory testing. Receiver?operating?curve analysis compared fasting C?peptide against known insulin resistance measures such as fasting plasma glucose, serum insulin, HOMA?IR, quantitative?insulin?sensitivitycheck? index, and metabolic syndrome for the prediction of cardiovascular and overall death. Subjects were then stratified by quartiles of C?peptide levels.
Cox proportional?hazards modeling compared hazards of cardiovascular and overall death amongst C?peptide quartiles and adjusted for potential confounders of cardiovascular and diabetes risk. Fasting serum C?peptide levels predicted cardiovascular and overall death better than other studied measures (AUC=0.62 and 0.60 respectively vs the rest, with AUC≤0.58 and ≤ 0.57 respectively, P<0.001). When compared with the lowest C?peptide quartile, subjects in the highest quartile had significantly higher adjusted hazard ratios (HR) of cardiovascular death (HR=1.60, 95%CI 1.07 to 2.39) and overall mortality (HR=1.72, 95%CI 1.34 to 2.21) after controlling for confounders.
Endothelial dysfunction [9] plays a key role in the initiation of cellular events evolving into the development of vascular complications in diabetes and hypertension. Diminished production and function of endothelium-derived nitric oxide and other vasoprotective factors and/or the exaggerated production of proinflammatory and vasoconstrictors such as angiotensin II, endothelin-1, reactive oxygen species, and cyclooxygenasederived metabolites of arachidonic acid eventually lead to endothelial dysfunction, resulting in elevated vascular tone which contributes to hypertension, vascular, and cardiac remodeling, culminating in micro vascular, macro vascular, and renal damages. Specific therapies targeting reactive oxygen species using antioxidants and inhibitors of the rennin-angiotensin system or increasing endothelial nitric oxide synthase activity might assist to reverse endothelial dysfunction and thus reduce the related cardiovascular morbidity and mortality in diabetes and hypertension.
Hypertension is a disruption of the endothelium [10], responsible for vascular tone. In hypertension and heart failure, the balance in the endothelial production of vasodilation and vasoconstriction mediators is altered. The release of both relaxing and contracting factors that modulate vascular smooth muscle tone participate in the pathophysiology of essential hypertension [11]. Endotheliumdependent vasodilation is regulated primarily by nitric oxide but also by an unidentified endothelium-derived hyperpolarizing factor and by prostacyclin. Endothelium-derived contracting factors include endothelin-1, vasoconstrictor prostanoids, angiotensin II and superoxide anions. Under physiological conditions, there is a balanced release of relaxing and contracting factors. The balance can be altered in cardiovascular diseases such as hypertension, atherosclerosis, diabetes and other conditions, thereby contributing to further progression of vascular and endorgan damage. In particular, endothelial dysfunction leading to decreased bioavailability of nitric oxide impairs endotheliumdependent vasodilation in patients with essential hypertension and may also be a determinant for the premature development of atherosclerosis.
In hypertensive patients and in animal models of hypertension, endothelium-dependent relaxations are impaired. However, this endothelial dysfunction presents different characteristics depending on the model studied. In Dahl-salt-sensitive rats, the decrease in endothelium-dependent relaxations is associated with impaired constitutive nitric oxide synthase activity. The presence of an endogenous nitric oxide synthase inhibitor and a decreased response of vascular smooth muscle to the mediator may contribute also to the dysfunction observed in this model. In large arteries from SHR, endothelium-dependent relaxations are impaired mainly because of the concomitant augmented release of endoperoxides activating thromboxane-endoperoxide receptors. Superoxide anions may also play a role in some models, but only in the early phase of the disease: whether or not these species contribute to further development of endothelial dysfunction or to increases in blood pressure remains to be examined. Different mechanisms of reduced nitric oxide activity have been shown both in hypertensive states and several cardiovascular diseases, and endothelial dysfunction is likely to occur prior to vascular dysfunction. Thus, the strategies currently used to improve endothelial dysfunction may result in decreased morbidity and mortality in hypertensive patients.
The endothelium has a strategical anatomical position between the circulating blood and vascular smooth muscle cells [12], and plays a primary role in the local modulation of vascular function and structure [13] by the production and release of several substances including nitric oxide and endothelins (ET). Endothelial cells play an important regulatory role in the circulation. The cells metabolize or activate vasoactive hormones; produce substances involved in coagulation, and can release endothelium-derived relaxing factors and contracting factors. Nitric oxide and prostacyclin are vasodilators and inhibitors of platelet function and NO is a labile substance produced from the catabolism of L-arginine. Nitric oxide and not only causes vessel relaxation, but also inhibits platelet aggregation, smooth muscle cell proliferation, monocyte adhesion, adhesion molecules expression and endothelin-1 (ET-1) production.
Endothelin is the most potent vasoconstrictor substance known. Endothelium-derived ET-1 is a potent vasoconstrictor and has inotropic and mitogenic properties. ET-1 acts through smooth muscle ET(A) and ET(B) receptors, which mainly mediate vasoconstriction, and endothelial ET(B) receptors, which oppose ET(A)- and ET(B)- mediated vasoconstriction by stimulating nitric oxide formation. Thus, the endothelium can profoundly affect platelet adhesion and aggregation, vascular smooth muscle tone and possibly also vascular smooth muscle growth. Under physiological conditions, endothelium-derived relaxing factors appear to dominate. In contrast, in hypertensive and atherosclerotic arteries the release of endothelium-derived relaxing factors and/or the responsiveness of vascular smooth muscle cells to the relaxing factors is reduced, while that of endothelium-derived contracting factors is augmented. This imbalance of endothelium-derived relaxing and contracting factors may be important in the pathogenesis of hypertension and its cardiovascular complications.
Both nitric oxide and ET-1 play a crucial role in the cardiovascular physiology and an alteration of these systems can be a promoter of or be associated with most cardiovascular diseases. Essential hypertension is a pathological condition characterized by endothelial dysfunction. In hypertensive patients nitric oxide availability is impaired because of the production of cyclooxygenase-derived vasoconstrictor substances. The latter may also mediate the vasoconstrictor response to exogenous ET-1 because in forearm circulation of essential hypertensives, but not of normotensive controls, the ET-1-induced vasoconstriction is significantly blunted by intrabrachial indomethacin.
Therefore, in normotensive subjects and essential hypertensives the vasoconstrictor effect of ET-1 seems to be dependent on different mechanisms. Moreover, in the peripheral circulation of normotensive subjects, where tonic nitric oxide production is preserved, unselective ET (A/B), receptor blockade by TAK- 044 causes a very modest degree of vasodilation. In contrast in essential hypertensives, where the tonic nitric oxide production is reduced, the vasodilating effect of TAK-044 is more evident, indicating that the predominant vascular effect of endogenous ET-1 is the vasoconstriction. A possible explanation for this finding, in addition to an increased production of the peptide, could be related to a reduced ET (B) receptor-mediated nitric oxide activation. These peculiar aspects of the role of ET-1 in essential hypertension could have physiopathological relevance.
Cardiovascular diseases (CVDs) are the major causes of mortality in persons with diabetes, and many factors, including hypertension, contribute to this high prevalence of CVD. The subject of diabetes mellitus as a comorbid disease that frequently confounds hypertension, adding significantly to its overall morbidity and mortality, will be updated in the present review. Hypertension is approximately twice as frequent in patients with diabetes compared with patients without the disease. Conversely, recent data suggest that hypertensive persons are more predisposed to the development of diabetes than are normotensive persons.
Furthermore, up to 75% of CVD in diabetes may be attributable to hypertension, leading to recommendations for more aggressive treatment (ie, reducing blood pressure to >130/85 mm Hg) in persons with coexistent diabetes and hypertension. Other important risk factors for CVD in these patients include the following: obesity, atherosclerosis, dyslipidemia, microalbuminuria, endothelial dysfunction, platelet hyperaggregability, coagulation abnormalities, and “diabetic cardiomyopathy.” The cardiomyopathy associated with diabetes is a unique myopathic state that appears to be independent of macrovascular/ microvascular disease and contributes significantly to CVD morbidity and mortality in diabetic patients, especially those with coexistent hypertension.
This update13 reviews the current knowledge regarding these risk factors and their treatment, with special emphasis on the cardiometabolic syndrome, hypertension, microalbuminuria, and diabetic cardiomyopathy. This update also examines the role of the renin-angiotensin system in the increased risk for CVD in diabetic patients and the impact of interrupting this system on the development of clinical diabetes as well as CVD.
Heart rate variability (HRV) is a useful noninvasive tool to assess cardiac autonomic function. The purpose of this study was to (1) compare measures of HRV between hypertensive and normotensive subjects and (2) examine the role of HRV as a predictor of new-onset hypertension. The first 2 hr of ambulatory ECG recordings obtained from 931 men and 1111 women attending a routine examination at the Framingham Heart Study were processed for HRV. Three time-domain and 5 frequencydomain variables were studied: standard deviation of normal RR intervals (SDNN), percentage of differences between adjacent normal RR intervals exceeding 50 milliseconds, square root of the mean of squared differences between adjacent normal RR intervals, total power (0.01 to 0.40 Hz), high frequency power (HF, 0.15 to 0.40 Hz), low frequency power (LF, 0.04 to 0.15 Hz), very low frequency power (0.01 to 0.04 Hz), and LF/HF ratio. On crosssectional analysis, HRV was significantly lower in hypertensive men and women.
Among 633 men and 801 women who were normotensive at baseline (systolic blood pressure <140 mm Hg and diastolic blood pressure < 90 mm Hg and not receiving antihypertensive treatment), 119 men and 125 women were newly hypertensive at follow-up 4 years later. After adjustment for factors associated with hypertension, multiple logistic regression analysis revealed that LF was associated with incident hypertension in men (odds ratio per SD decrement [OR], 1.38; 95% confidence interval [CI], 1.04 to 1.83) but not in women (OR, 1.12; 95% CI, 0.86 to 1.46). SDNN, HF, and LF/HF were not associated with hypertension in either sex. HRV is reduced in men and women with systemic hypertension. Among normotensive men, lower HRV was associated with greater risk for developing hypertension. These findings are consistent with the hypothesis that autonomic dysregulation is present in the early stage of hypertension [14].
Resistant hypertension is a common clinical problem faced by both primary care clinicians and specialists. While the exact prevalence of resistant hypertension is unknown, clinical trials suggest that it is not rare, involving perhaps 20% to 30% of study participants. As older age and obesity are 2 of the strongest risk factors for uncontrolled hypertension, the incidence of resistant hypertension will likely increase as the population becomes more elderly and heavier [15]. The prognosis of resistant hypertension is unknown, but cardiovascular risk is undoubtedly increased as patients often have a history of long-standing, severe hypertension complicated by multiple other cardiovascular risk factors such as obesity, sleep apnea, diabetes, and chronic kidney disease. The diagnosis of resistant hypertension requires use of good blood pressure technique to confirm persistently elevated blood pressure levels. Lack of blood pressure control secondary to poor medication adherence must be excluded. Resistant hypertension is multifactorial in etiology.
Successful treatment requires identification and reversal of lifestyle factors contributing to treatment resistance; diagnosis and appropriate treatment of secondary causes of hypertension; and use of effective multidrug regimens. Recommendations for the pharmacological treatment of resistant hypertension remain largely empiric due to the lack of systematic assessments of 3 or 4 drug combinations. Studies of resistant hypertension are limited by the high cardiovascular risk of patients within this subgroup, which generally precludes safe withdrawal of medications; the presence of multiple disease processes (e.g., sleep apnea, diabetes, chronic kidney disease, atherosclerotic disease) and their associated medical therapies, which confound interpretation of study results; and the difficulty in enrolling large numbers of study participants.
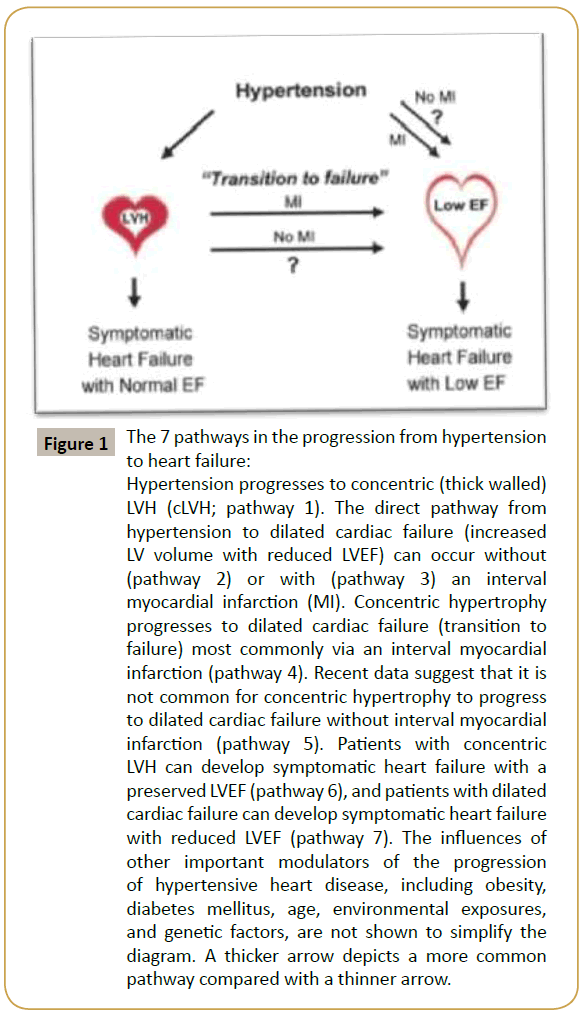
Hypertensive heart disease is a constellation of abnormalities that includes left ventricular hypertrophy (LVH), systolic and diastolic dysfunction, and their clinical manifestations including arrhythmias and symptomatic heart failure [16,17]. The classic paradigm of hypertensive heart disease is that the left ventricular (LV) wall thickens in response to elevated blood pressure as a compensatory mechanism to minimize wall stress. LVH is defined as an increase in LV mass. Criteria to define LVH require using a somewhat arbitrary threshold to dichotomize a trait that appears to have a continuous relationship with subsequent risk [18]. Subsequently, after a series of poorly characterized events (“transition to failure”), the left ventricle dilates, and the LV ejection fraction (EF) declines (defined herein as “dilated cardiac failure”). The purpose of this review is to focus on the key steps in the progression of hypertensive heart disease (Figure 1), highlighting recent advances as well as some unresolved controversies.
Figure 1: The 7 pathways in the progression from hypertension to heart failure:
Hypertension progresses to concentric (thick walled)LVH (cLVH; pathway 1). The direct pathway from hypertension to dilated cardiac failure (increased LV volume with reduced LVEF) can occur without (pathway 2) or with (pathway 3) an interval myocardial infarction (MI). Concentric hypertrophy progresses to dilated cardiac failure (transition to failure) most commonly via an interval myocardial infarction (pathway 4). Recent data suggest that it is not common for concentric hypertrophy to progress to dilated cardiac failure without interval myocardial infarction (pathway 5). Patients with concentric LVH can develop symptomatic heart failure with a preserved LVEF (pathway 6), and patients with dilated cardiac failure can develop symptomatic heart failure with reduced LVEF (pathway 7). The inÃÆïÃâìÃâââ¬Å¡uences of other important modulators of the progression of hypertensive heart disease, including obesity, diabetes mellitus, age, environmental exposures, and genetic factors, are not shown to simplify the diagram. A thicker arrow depicts a more common pathway compared with a thinner arrow.
References
- Richard E Klabunde, Cardiovascular Physiology Concepts.
- Albert W Dreisbach, Pathophysiology of Hypertension.
- Piper MA, Evans CV, Burda BU, Margolis KL, O’Connor E (2014) Screening for High Blood Pressure in Adults: A Systematic Evidence Review for the US Preventive Services Task Force. Evidence Synthesis No: 121. AHRQ Publication No: 13-05194-EF-1.
- New Concepts in Hypertension Therapy and Cardiovascular Disease.
- Dominiczak AF, Jeffs B, Connell JM (1998) New genetic concepts in hypertensive cardiovascular disease. Curr Opin Cardiol 13: 304-311.
- Thomas Unger. The role of the renin-angiotensin system in the development of cardiovascular disease.
- Ralph A DeFronzo, and Eleuterio Ferrannini (1991) Insulin Resistance: A Multifaceted Syndrome Responsible for NIDDM, Obesity, Hypertension, Dyslipidemia, and Atherosclerotic Cardiovascular Disease. Diabetes Care March 14: 173-194.
- Nileshkumar Patel, Tracey H Taveira, Gaurav Choudhary, Hilary Whitlatch, Wen Chih Wu (2012) Fasting Serum C?Peptide Levels Predict Cardiovascular and Overall Death in Nondiabetic Adults. J Am Heart Assoc 1: e003152.
- Wong WT, Wong SL, Tian XY, Huang Y (2010) Endothelial dysfunction: the common consequence in diabetes and hypertension. J Cardiovasc Pharmacol 55: 300-307.
- Boulanger CM (1999) Secondary endothelial dysfunction: hypertension and heart failure. J Mol Cell Cardiol 31: 39-49.
- Puddu P, Puddu GM, Zaca F, Muscari A (2000) Endothelial dysfunction in hypertension. Acta Cardiol 55: 221-232.
- Lüscher TF (1990) Imbalance of endothelium-derived relaxing and contracting factors. A new concept in hypertension? Am J Hypertens 3: 317-330.
- Taddei S, Virdis A, Ghiadoni L, Salvetti A (2000) Vascular effects of endothelin-1 in essential hypertension: relationship with cyclooxygenase-derived endothelium-dependent contracting factors and nitric oxide. J Cardiovasc Pharmacol 35: S37-S40.
- James R Sowers, Murray Epstein, Edward D Frohlich (2001) Diabetes, Hypertension, and Cardiovascular Disease An Update. Hypertension 37: 1053-1059.
- Jagmeet P Singh, Martin G Larson, Hisako Tsuji, Jane C Evans, Christopher J, et al. (1998) Reduced Heart Rate Variability and New-Onset Hypertension: Insights into Pathogenesis of Hypertension: The Framingham Heart Study. Hypertension 32: 293-297.
- Thomas D Giles, Bonita Falkner, Robert M Carey Toto, Anthony White, William C Cushman, et al. (2008) Statement for High Blood Pressure Research From the American Heart Association Professional Education Committee of the Council Resistant Hypertension: Diagnosis, Evaluation, and Treatment: A Scientific. Hypertension 51: 1403-1419.
- Mark H Drazner (2011) The Progression of Hypertensive Heart Disease. Circulation 123: 327-334.
- Schillaci G, Verdecchia P, Porcellati C, Cuccurullo O, Cosco C, et al. (2000) Continuous relation between left ventricular mass and cardiovascular risk in essential hypertension. Hypertension 35: 580-586.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences