Effect of Aerobic Exercise Training on Cgmp Levels and Blood Pressure in Medicated Hypertensive Postmenopausal Women (Cgmp Levels and Blood Pressure in Women)
Novais IP, Jarrete AP, Puga GM, Araujo HN, Fernandes RA, Delbin MA and Zanesco A
1Laboratory of Cardiovascular Physiology and Exercise Science, Institute of Biosciences, UNESP, Rio Claro/SP, Brazil
2Department of Physical Education, UNESP, Presidente Prudente/SP, Brazil
3Department of Structural and Functional Biology, Institute of Biology, UNICAMP, Campinas/SP, Brazil
- Corresponding Author:
- Angelina Zanesco
Professor in Physiology, Institute of Biosciences
UNESP, Av-24A, 515-Bela Vista, Rio Claro/SP, Brazil
Tel: 55-1935264324
Fax: 55-1935264321
E-mail: azanesco@rc.unesp.br
Received Date: December 10, 2015 Accepted Date: December 13, 2015 Published Date: December 23, 2015
Citation: Novais IP, Jarrete AP, Puga GM, et al. Effect of Aerobic Exercise Training on Cgmp Levels and Blood Pressure in Medicated Hypertensive Postmenopausal Women(Cgmp Levels and Blood Pressure in Women). Insights Blood Press 2015, 2:1.
Abstract
The second messenger cyclic guanosine 3’5’monophosphate (cGMP) has been in the focus of therapeutic target in a variety of disorders such as erectile dysfunction, arterial hypertension, atherosclerosis and heart failure. Nevertheless, a recent study has shown that cGMP activators are less efficient in estrogen deficiency animals. Thus, studies involving non-pharmacological approach examining NO/ cGMP signaling pathway in hypertensive postmenopausal women are clinically relevant. Therefore, we examined the concentrations of NO/cGMP, redox state and blood pressure in medicated hypertensive postmenopausal women (HT, n=28) at baseline and after aerobic exercise training (AET) comparing with normotensive group (NT, n=33). AET consisted of 24 sessions in treadmill, 3 times/week, 30-40 min for each session. Nitrite/nitrate, cGMP levels and redox state (SOD and catalase activity and TBARS levels) were similar between the two groups at baseline. In medicated HT group, AET was effective in increasing cGMP concentrations (28%), accompanied by an up-regulation of superoxide dismutase (SOD) and catalase activity, 97 and 37%, respectively. In NT group, we found an increase only in SOD activity (58%). In conclusion, our findings show that AET is an effective non-pharmacological approach in increasing cGMP levels as well as up-regulating antioxidant in medicated hypertensive postmenopausal women. It should also be emphasized that these findings provide information on the circulating biomarkers that might delay the developing of cardiovascular events in this particular population.
Keywords
Arterial hypertension; Physical exercise; Blood pressure; Postmenopausal women
Introduction
The second messenger cyclic guanosine 3’5’monophosphate (cGMP) has been in the focus of therapeutic target in a variety of disorders such as erectile dysfunction, arterial hypertension, atherosclerosis and heart failure. Indeed, agents that increase cGMP generation or decrease its degradation have been intensively studied by different laboratories such as inhibitors of phosphodiesterase 5 (PDE5) and activators of soluble guanylyl cyclase [1,2]. Thus, strategies to enhance cGMP levels would be useful in management cardiovascular diseases (CVD) or in preventing their complications. Of note, it was recently demonstrated that the efficacy of PDE5 inhibition in cardiac diseases is dependent of estrogen levels in female mice [3]. Thus, clinical trial regarding the efficacy of some compounds on cardiac diseases should be carefully evaluated in women after menopause since estrogen deficiency has been pointed out as the main cause of increased prevalence of CVD in this period of life in this population.
On the other hand, it has been largely shown that aerobic exercise training (AET) is an important approach to prevent or to mitigate the complications of CVD promoting an improvement of eNOS/NO signaling pathway in both humans and laboratory animals [4,5]. Interestingly, only two studies exist evaluating the effects of aerobic exercise training on cGMP levels in women after menopause; however, the number of the participants were too small as well as age-matched group is necessary [6,7]. Thus, the objective of this study was to examine the effects of AET on cGMP levels in hypertensive postmenopausal women pharmacologically controlled with antihypertensive therapy. The rationale for that is most of hypertensive patients are encouraged by physician to perform exercise during pharmacological treatments; thus, it would be feasible to evaluate medicated participants in an attempting to detect additional effects of both therapy (AET plus medication). We also compared medicated hypertensive postmenopausal women with normotensive and the effects of AET on blood pressure and its association with cardiovascular biomarkers.
Materials and Methods
Participants
The study involved 61 postmenopausal women, 33 subjects represented a group of normotensive participants (NT group) and 28 medicated hypertensive women (HT group) according to previous medical diagnosis. The inclusion criteria were: reported absence of menses for at least 1 year at recruitment (natural menopause), body mass index ≤ 30 kg/m2, and physically inactive (<150 minutes of moderate physical activity per week or < 60 minutes of vigorous physical activity per week). Exclusion criteria for participation in the study were: current use of menopausal hormone therapy (MHT), smoker, individuals with diabetes (type 1 or 2), previous CVD (stroke, heart failure), renal dysfunction, inability to perform physical exercise, poorly controlled hypertension [8], and mental illnesses.
Participants were instructed to maintain their habitual diet routine during the protocol. The study was approved by the Ethical Committee of the Bioscience Institute of the University of São Paulo State (Ref. 4395/2010) and each participant signed a written informed consent.
Experimental Design
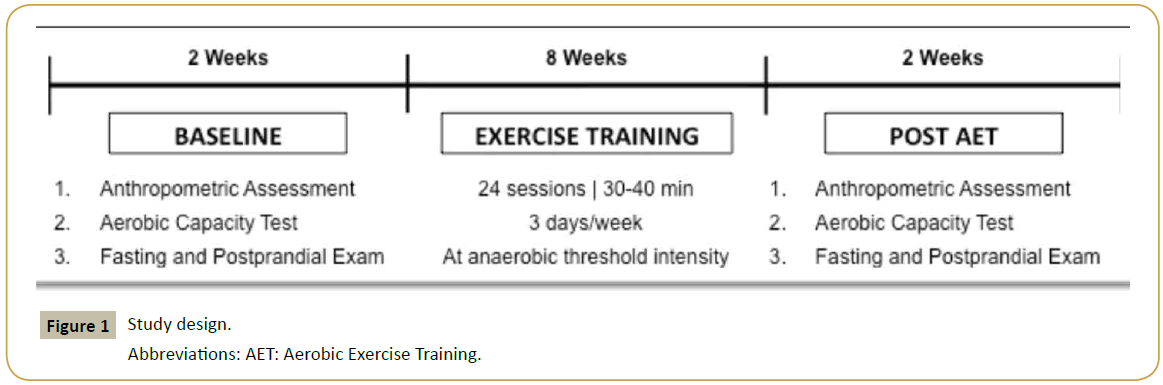
Participants of the study underwent to 24-session of AET, see Figure 1 for more details. Anthropometric assessment (height, weight, body mass index), aerobic capacity test, biochemical analyses were performed at baseline and after AET.
Anthropometric and cardiovascular assessments
Body weight (kg) and height (cm) were determined using a scale and stadiometer (Toledo® 2096 PP) and body mass index was calculated (BMI in kg/m2). Blood pressure (BP) was performed using semi-automatic equipment (Microlife® MIB-P3BTOA) and three BP measurements were taken. Resting BP was determined as the average of the measurements. Heart rate (HR) was assessed using a heart rate monitor (Polar® FT1 TRQ). Both BP and HR were evaluated after 20 minutes in the sitting position. All measurements were taken following a validated protocol [9].
Aerobic Capacity Test
Before the exercise tests and training program, women were familiarized to the treadmill by walking during 2-3 days, depending on each participant. After that, all participants performed 2-5 tests to determine the maximal lactate steady state (MLSS) to be used for the intensity of AET prescription. Each constant workload test with fixed walking speed (5.5 Km/h) on a treadmill (Movement® RT 250 PRO) lasted 30 min according to previous study [10]. The intensity was controlled by the inclination of the treadmill (measured in %), with the grade adjusted according to the aerobic capacity of the participant in each session. The inclination ranged between 2% and 10% during the test sessions. Capillary blood samples (25 μL) were collected by micropuncture (earlobe) and then stored in microcentrifuge tubes containing 400 μL Trichloroacetic acid (4%) at minutes 10 and 30 for the determination of blood lactate concentration ([Lac]), [11]. The MLSS workload (anaerobic threshold) was defined as the highest power intensity (treadmill inclination) at which blood lactate concentration did not increase by more than 1 nmol/L between minutes 10 and 30 of the constant load test [10].
Blood collection and biochemical analyses
Blood samples were collected at 07 h 30 .m. in two different days: after 12 hrs of overnight fast (fasting) and after daily breakfast (postprandial). Blood samples were immediately centrifuged at 3000 rpm during 10 min and the supernatant (plasma and serum) was separated into several aliquots and stored at -80°C for future analyses.
Nitrite/Nitrate (NOx-) and cGMP levels
Plasma concentrations of NOx - were measured to evaluate NO production by commercial available kit (Cayman Chemical®, Ann Arbor, MI, USA). Before starting this assay, samples were ultrafiltered through micro filter (Microcon® Centrifugal Filter Units, 10 kDa, Millipore, Bedford, MA). Plasma concentrations of cGMP were measured by ELISA kit using a commercial available kit (Cayman Chemical®, Ann Arbor, MI, USA).
Antioxidant enzyme activities and lipid peroxidation
Superoxide Dismutase (SOD) and catalase (CAT) activity were measured by ELISA using a commercial available kit (Cayman Chemical®, Ann Arbor, MI, USA). SOD’s assay detects radicals superoxide generated by xanthine oxidase and hypoxanthine, revealing the plasmatic activity of this enzyme. Catalase’s assay is based on the reaction of the enzyme with methanol in an optimal H2O2 concentration. Levels of Thiobarbituric Acid Reactive Substances (TBARS) were determined by using the TBARS assay kit again from Cayman Chemical® (Ann Arbor, MI, USA) in which the MDA-TBA adduct formed by the reaction of malondialdehyde (MDA) and thiobarbituric acid (TBA) under high temperature (90–100ºC) and acidic conditions is measured colorimetrically at 530–540nm. This method reflects lipid peroxidation and was expressed in μM MDA.
Aerobic exercise training (AET)
The AET program began with appropriate warm up (5 min speed walking 4 km/h on a treadmill without inclination), and all the exercise sessions were supervised by cardiovascular physiologists. Participants performed the exercise session on a treadmill in a quiet room with environment-controlled temperature (≈25°C) and humidity (≈50%). Exercise was performed 3 days/week, for a total of 8 weeks (24 sessions), and each session consisting of 30 min (first 3 weeks), 35 min (following 3 weeks) and, finally, 40 min (last 2 weeks). The intensity (treadmill inclination) of exercise was prescribed according to previous individual MLSS test. Heart rate and the rate of perceived exertion (Borg's scale) were also recorded every 10 min in each session.
Statistical analysis
Data are presented as mean ± standard error mean. Before applying statistical methods, the normality of the data was tested by the Kolmogorov-Smirnov’s test. Two-way analysis of variance (ANOVA) was used comparing the effect of AET and difference between groups (NT and HT). Bonferroni correction was used as a post hoc test. The power of the study was 95% for both groups. Linear regression models (expressed as β and its 95% confidence intervals [95%CI]) were created in order to analyze the effect of potential confounders (chronological age, years after menopause diagnosis and modifications in BMI) in the differences attributed to AET. Statistical analyses were performed using the Graphpad Prism® version 5 software packages (California, USA) and the level of significance was set at p<0.05.
Results
Sixty-one postmenopausal women were enrolled in the study (NT=33 and HT=28). Table 1 shows the characteristics of the participants. Both groups were homogenous in terms of time after menopause, body mass index, blood pressure values and heart rate. Approximately 70% of medicated hypertensive women were on angiotensin system inhibition plus diuretics and 30% were on beta blocker/calcium channel blocker. Exercise training did not affect the body mass index as well as cardiovascular parameters.
| NT (n=33) | HT (n=28) | |||
|---|---|---|---|---|
| Baseline | Post AET | Baseline | Post AET | |
| Age (years) | 55.6 ± 0.8 | 57.2 ± 1.0 | ||
| Body Mass Index (kg/m2) | 27.1 ± 0.6 | 27.1 ± 0.6 | 28.2 ± 0.5 | 28.0 ± 0.5 |
| Systolic BP (mmHg) | 113.6 ± 2.0 | 112.6 ± 2.1 | 117.3 ± 2.0 | 115.3 ± 2.1 |
| Diastolic BP (mmHg) | 70.4 ± 1.4 | 69.7 ± 1.3 | 73.2 ± 1.8 | 69.9 ± 2.0 |
| Heart Rate (bpm) | 72.7 ± 1.6 | 69.7 ± 1.8 | 69.3 ± 1.9 | 67.7 ± 1.8 |
| Anti-hypertensive therapy (n): | ||||
| AT1 blocker | 6 | |||
| ACE inhibitor | 2 | |||
| β-blocker | 1 | |||
| Diuretic | 2 | |||
| Diuretic + AT1blocker | 5 | |||
| Diuretic + ACE | 2 | |||
| Diuretic + β-blocker | 2 | |||
| Diuretic + CCB | 1 | |||
| AT1 + CCB | 1 | |||
| AT1 + β-B | 1 | |||
| Diuretic + β-B + ACE | 2 | |||
| Diuretic + β-B + CCB | 1 | |||
| Diuretic + AT1 + CCB | 2 | |||
Table 1: Characteristics of postmenopausal women at baseline and post aerobic exercise training.
Table 2 shows biochemical parameters in both groups. Exercise training for 24 sessions was effective in increasing the exercise intensity without changes in blood lactate concentration, approximately 38% and 35% for normotensive and hypertensive women, respectively, showing the efficacy of the training intensity in improving aerobic capacity. Lipid profile and blood glucose were also similar between normotensive and hypertensive postmenopausal women at baseline, and exercise training did not modify these parameters.
| NT (n=33) | HT (=28) | |||
|---|---|---|---|---|
| Baseline | Post AET | Baseline | Post AET | |
| Intensity (treadmill inclination %) | 5.7 ± 0.4 | 7.9 ± 0.5a | 5.0 ± 0.4 | 6.8 ± 0.4a |
| [Lac] at Anaerobic Threshold (µM) | 2.9 ± 0.2 | 3.1 ± 0.2 | 3.3 ± 0.3 | 3.0 ± 0.3 |
| Total Cholesterol (mg/dl) | 210.0 ± 5.2 | 204.3 ± 4.6 | 199.4 ± 5.8 | 189.7 ± 4.5 |
| C-HDL (mg/dl) | 45.6 ± 1.6 | 48.3 ± 1.4 | 47.4 ± 1.3 | 48.3 ± 1.2 |
| C-LDL (mg/dl) | 137.0 ± 5.5 | 130.6 ± 4.3 | 126.9 ± 5.7 | 119.3 ± 4.4 |
| C-VLDL (mg/dl) | 26.5 ± 2.1 | 25.5 ± 1.7 | 25.1 ± 1.6 | 22.0 ± 1.4 |
| Triglycerides (mg/dl) | 132.5 ± 10.5 | 127.5 ± 8.6 | 125.6 ± 8.1 | 110.0 ± 7.2 |
| Blood glucose (mg/dl) | 87.3 ± 1.6 | 90.8 ± 1.6 | 88.0 ± 1.6 | 90.7 ± 1.9 |
aSignificantly different compared with respectively baseline (p < 0.05).
Table 2: Aerobic capacity test and biochemical parameters in normotensive and hypertensive postmenopausal women at baseline and post aerobic exercise training.
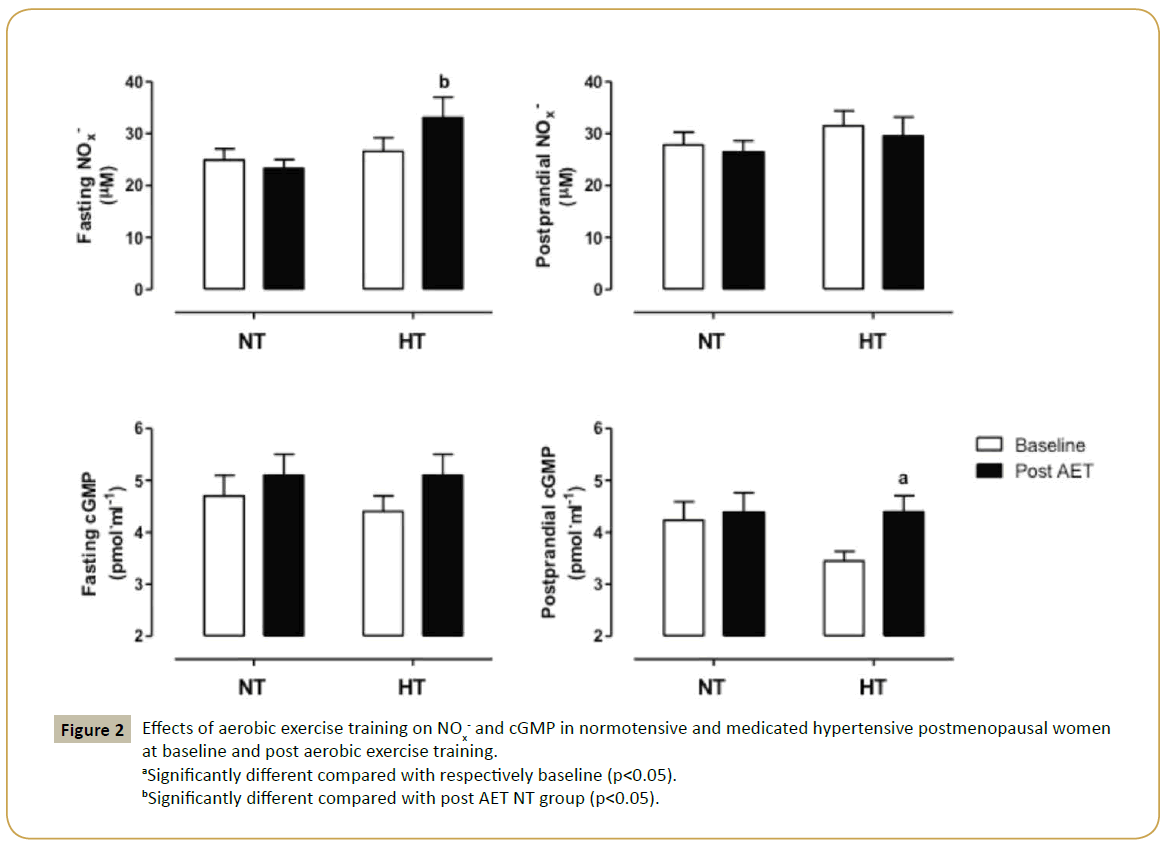
Since experimental studies have attributed to the greater prevalence of arterial hypertension in women after menopause to endothelial dysfunction, we evaluated NO/cGMP signaling pathway in both groups. We found no differences in NOx- and cGMP concentrations between normotensive and hypertensive women at baseline. Interestingly, trained medicated hypertensive group had increased cGMP concentration (28%), but no changes in NOx- levels were observed in postprandial state. In fasting state, we found a significant difference in NOx- levels between trained groups, approximately 42%, while no changes were detected in cGMP concentrations in trained women. Figure 2 illustrates these data.
Figure 2: Effects of aerobic exercise training on NOx- and cGMP in normotensive and medicated hypertensive postmenopausal women at baseline and post aerobic exercise training.
aSignificantly different compared with respectively baseline (p<0.05).
bSignificantly different compared with post AET NT group (p<0.05).
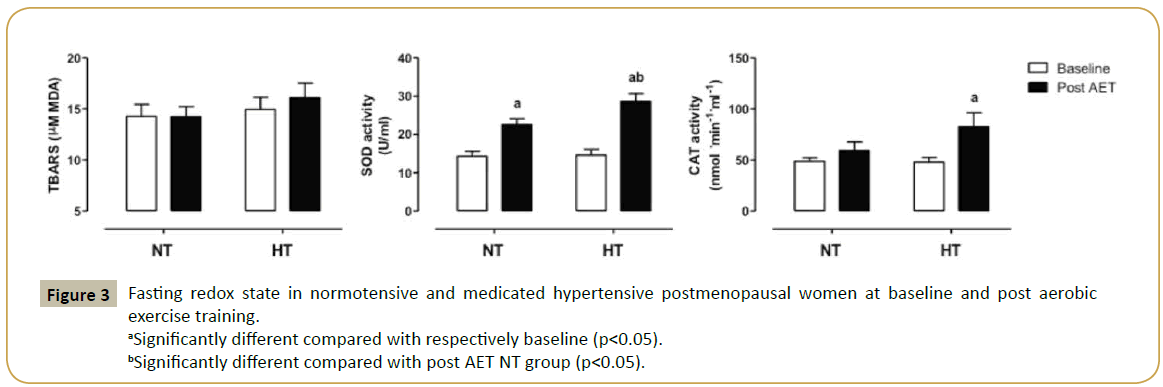
We also examined fasting redox state by measuring TBARS concentration and antioxidant enzymes in plasma. At baseline, no differences were found between the groups for all parameters. Exercise training did not change TBARS concentration that reflects lipid peroxidation, whereas SOD activity was significantly increased in both groups (58 and 97% for normotensive and medicated hypertensive women, respectively). Moreover, trained medicated hypertensive postmenopausal women presented higher SOD activity as compared with NT group, approximately 27%. Interestingly, catalase activity was significantly increased in trained medicated hypertensive women, approximately 73% (Figure 3).
In the multivariate models, modifications in cGMP in trained medicated hypertensive group were significantly related to years after menopause diagnosis (β= -0.093 [95%CI= -0.169 to -0.018]). Similarly, modifications in catalase activity were significantly related to years after menopause diagnosis (β= -4.748 [95%CI= -9.423 to -0.073]) in trained medicated hypertensive group. On the hand, modifications in SOD activities and BMI were not related to any of the potential confounders previously selected.
Discussion
Our findings clearly show that medicated hypertensive postmenopausal women are more responsive to AET in increasing NO/cGMP signaling pathway as compared with normotensive group even though both groups were homogenous regarding cardiovascular biomarkers and well-controlled blood pressure at baseline.
Arterial hypertension is a chronic disease and uncontrolled blood pressure can lead to a variety of complications such as vascular remodeling, heart attack, stroke and heart failure [12]. In addition, the Global Burden of Disease Study identified elevated blood pressure as the leading risk factor, among 67 studied, for death and disability- adjusted life-years lost during 2010 [13]. Moreover, it has shown that the incidence of CVD in women increases after menopause [14]. It is believed that sex hormones play a major role in this phenomenon and estrogen deficiency might be the primary cause of this phenomenon in women in climacteric period; however, most of studies are based on experimental studies, using ovariectomized animals or isolated cells [15]. On the other hand, clinical trials have shown that menopausal hormone therapy, mainly the oral administration of estrogen plus progestin compared with placebo, increases risks of CVD such as coronary heart disease, stroke, and thromboembolic complications [16]. More recently, studies have shown that exogenous intranasal administration of estradiol promoted an improvement of relaxing responses in healthy postmenopausal women [17,18]. Thus, pharmacological and non-pharmacological approaches to control blood pressure or to prevent its complication in women are clinically relevant. In our study, we found that AET was effective in promoting increase in cardiovascular biomarkers that are crucial for blood pressure regulation or for reducing oxidative stress, even though no changes in blood pressure values were found in medicated hypertensive women. Indeed, it has been reported that exercise training is more effective in lowering blood pressure in uncontrolled hypertensive subjects [19]. Moreover, epidemiological studies have systematically shown the beneficial effects of physical activity/exercise on CVD as well as how higher fitness levels could delay the developing of arterial hypertension or its complications [20,21]. Thus, our findings are clinically relevant since most of medicated hypertensive patients are encouraged to get involved in physical activities or in supervised exercise training. Furthermore, great enthusiasm exists regarding new pharmacological target to increase cGMP levels by increasing cGMP generation or decreasing its degradation mainly in patients with heart failure [2]. Nevertheless, recent clinical trials data were disappointing mainly because of up-regulation of PDE5 during long-term inhibition treatment. Interestingly, our study shows that a medicated hypertensive postmenopausal woman is responsive in increasing cGMP levels after AET that would prevent or, so far, delay the developing of heart failure during climacteric period. The possible mechanism for that might be related to the effect of shear stress induced by physical exercise. Indeed, physical exercise is a powerful stimulus to promote vascular shear stress activating mechanosensors present in endothelial cells. These mechanosensors are coupled to complex biochemical signal pathways, such as Ras/MEK/ERK, c-Src, G proteins, ion channel, VE-cadherin, and PI3K/Akt, which in turn regulate NO/cGMP pathway [22,23]. Accordingly, the beneficial effects of physical training on cardiovascular system are strongly associated with increased blood flow [4].
In conclusion, our findings show that AET is an effective nonpharmacological approach in increasing cGMP levels as well as up-regulating antioxidant enzymes in medicated hypertensive postmenopausal women. It should also be emphasized that these findings provide information on the circulating biomarkers that might delay the developing of cardiovascular events in this particular population.
References
- Stasch JP, Pacher P, Evgenov OV (2011) Soluble guanylatecyclase as an emerging therapeutic target in cardiopulmonary disease. Circulation. 123: 2263-2273.
- Buglioni A, Burnett JC Jr (2015) NewPharmacologicalStrategies to IncreasecGMP. Annu Rev Med.Urology 75: 961-967.
- Sasaki H, Nagayama T, Blanton RM, Seo K, Zhang M, et al. (2014) PDE5 inhibitor efficacy is estrogen dependent in female heart disease. J Clin Invest 124: 2464-2471.
- Zanesco A, Antunes E (2007) Effects of exercisetraining on the cardiovascular system: pharmacologicalapproaches. PharmacolTher 114: 307-317.
- Claudino MA, Franco-Penteado CF, Priviero FB, Camargo EA, Teixeira SA, et al. (2010) Upregulation of gp91phox subunit of NAD(P)H oxidase contributes to erectile dysfunction caused by long-term nitric oxide inhibition in rats: reversion by regular physical training. Urology 75: 961-967.
- Maeda S, Tanabe T, Otsuki T, Sugawara J, Iemitsu M, Miyauchi T, Kuno S, Ajisaka R, Matsuda M. (2004) Moderate regular exercise increases basal production of nitric oxide in elderly women. Hypertens Res 27: 947-953.
- Jarrete AP, Novais IP, Nunes HA, Puga GM, Delbin MA,et al. (2014) Influence of aerobic exercise training on cardiovascular and endocrine-inflammatory biomarkers in hypertensive postmenopausal women. J ClinTranslEndocrinol 1: 108-114.
- James PA, Oparil S, Carter BL, Cushman WC, Dennison-Himmelfarb C, et al. (2014) Evidence-based guideline for the management of high blood pressure in adults: report from the panel members appointed to the Eighth Joint National Committee (JNC 8) JAMA 311: 507-520.
- SociedadeBrasileira de Cardiologia; SociedadeBrasileira de Hipertensão; SociedadeBrasileira de Nefrologia. (2010) [VI Brazilian Guidelines on Hypertension]. Arq Bras Cardiol 95: 1-51.
- Beneke R(2003) Methodologicalaspects of maximallactatesteadystate-implications for performancetesting. Eur J ApplPhysiol 89: 95-99.
- Engel PC, Jones JB (1978)Causes and elimination of erratic blanks in enzymatic metabolite assays involving the use of NAD+ in alkaline hydrazine buffers: improved conditions for the assay of L-glutamate, L-lactate, and other metabolites.Anal Biochem 88: 475-84.
- Borlaug BA, Paulus WJ (2011) Heart failure with preservedejectionfraction: pathophysiology, diagnosis, and treatment. EurHeart J 32: 670-9.
- Lim SS, Vos T, Flaxman AD(2012) A comparative risk assessment of burden of disease and injury attributable to 67 risk factors and risk factor clusters in 21 regions, 1990-2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 380(9859): 2224-60.
- Mozaffarian D, Benjamin EJ, Go AS, Arnett DK, Blaha MJ, et al. (2015) on behalf of the American Heart Association Statistics Committee and Stroke Statistics Subcommittee. Heart disease and stroke statistics-2015 update: a report from the American Heart Association. Circulation.
- Maranon R, Reckelhoff JF (2013) Sex and gender differences in control of blood pressure. ClinSci (Lond) 125: 311–318.
- Wassertheil-Smoller S, Hendrix SL, Limacher M, Heiss G, Kooperberg C, et al. (2003) Effect of estrogen plus progestin on stroke in postmenopausal women: the Women's Health Initiative: a randomized trial. JAMA 289: 2673–2684.
- Ciccone MM, Cicinelli E, Giovanni A, Scicchitano P, Gesualdo M, et al. (2012) Ophthalmic artery vasodilation after intranasal estradiol use in postmenopausal women. J AtherosclerThromb19:1061-1065.
- Ciccone MM, Scicchitano P, Gesualdo M, Fornarelli F, Pinto V, et al. (2013) Systemic vascular hemodynamic changes due to 17-β-estradiol intranasal administration. J CardiovascPharmacolTher. 18:354-358.
- Fagard RH (2006) Exercise is good for yourbloodpressure: effects of endurancetraining and resistance training. ClinExpPharmacolPhysiol 33: 853-856.
- Blair SN, Kohl HW IIIrd, Paffenbarger RS Jr, Clark DG, Cooper KH, et al. (1989) Physical fitness and all-cause mortality. A prospective study of healthy men and women. JAMA 262: 2395-2401.
- Liu J, Sui X, Lavie CJ, Zhou H, Park YM, et al.(2014) Effects of cardiorespiratoryfitness on bloodpressuretrajectory with aging in a cohort of healthymen. J Am CollCardiol 64: 1245-1253.
- Balligand JL, Feron O, Dessy C (2009) eNOS activation by physical forces: from short-term regulation of contraction to chronic remodeling of cardiovascular tissues. Physiol Rev 89:481-534.
- Benecke R (1995) Anaerobicthreshold, individualanaerobicthreshold, and maximallactatesteadystate in rowing. Med Sci Sports Exerc 27: 863-867.
Open Access Journals
- Aquaculture & Veterinary Science
- Chemistry & Chemical Sciences
- Clinical Sciences
- Engineering
- General Science
- Genetics & Molecular Biology
- Health Care & Nursing
- Immunology & Microbiology
- Materials Science
- Mathematics & Physics
- Medical Sciences
- Neurology & Psychiatry
- Oncology & Cancer Science
- Pharmaceutical Sciences